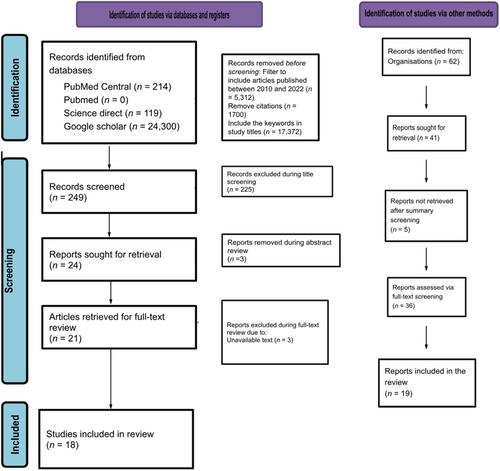
In response to the situation of the African healthcare system, the African Medicines Agency (AMA) was established by the African Union (AU) to regulate access to medicines and support the local manufacture of medications. This study aimed to describe the factors that enabled the establishment of the African Medicines Agency and its successes, challenges, and perceived benefits. We reviewed data sources that explored the progress and challenges of the African Medicines Agency and Medicines Regulation in Africa. The SPIDER framework was used to organise the research focus and to extract the keywords for the literature search. The study data were obtained from PubMed Central, ScienceDirect, and Google Scholar. Out of 249 studies screened, 19 were selected for this narrative review. Critical successes observed in the agency's establishment include the appointment of a Special Envoy, the selection of its headquarters, and the signing of its treaty by 37 member states. However, it is hindered by poor political commitment, differences in risk-benefits interpretation and organizational structure, weak legal and regulatory frameworks, inadequate financial mechanisms, and inadequate political and policy leadership in some member states. The value of AMA in achieving optimal health outcomes and its other benefits must be considered despite the challenges being encountered. Therefore, all member states should adopt the best procedures in signing and ratifying the treaty and implementing associated commitments to improve efficiency and accountability in African medicine regulation.