Correctly distinguishing between benign and malignant pulmonary nodules can avoid unnecessary invasive procedures. This study aimed to construct a deep learning radiomics clinical nomogram (DLRCN) for predicting malignancy of pulmonary nodules.
One thousand and ninety-eight patients with 6–30 mm pulmonary nodules who received histopathologic diagnosis at 3 centers were included and divided into a primary cohort (PC), an internal test cohort (I-T), and two external test cohorts (E-T1, E-T2). The DLRCN was built by integrating adipose tissue radiomics features, intranodular and perinodular deep learning features, and clinical characteristics for diagnosing malignancy of pulmonary nodules. The least absolute shrinkage and selection operator (LASSO) was used for feature selection. The performance of DLRCN was assessed with respect to its calibration curve, area under the curve (AUC), and decision curve analysis (DCA). Furthermore, we compared it with three radiologists. The net reclassification improvement (NRI), integrated discrimination improvement (IDI), and subgroup analysis were also taken into account.
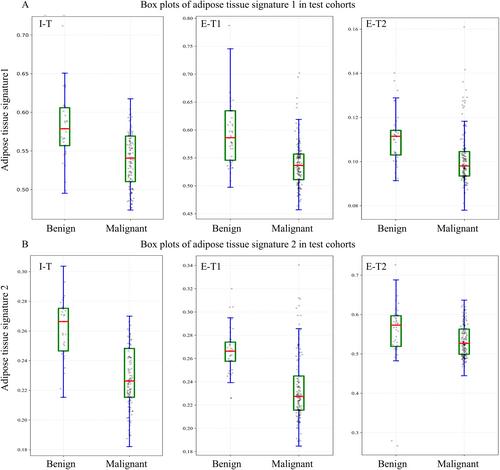
The incorporation of adipose tissue radiomics features led to significant NRI and IDI (NRI = 1.028, p < 0.05, IDI = 0.137, p < 0.05). In the I-T, E-T1, and E-T2, the AUCs of DLRCN were 0.946 (95% CI: 0.936, 0.955), 0.948 (95% CI: 0.933, 0.963) and 0.962 (95% CI: 0.945, 0.979), The calibration curve revealed good predictive accuracy between the actual probability and predicted probability (p > 0.05). DCA showed that the DLRCN was clinically useful. Under equal specificity, the sensitivity of DLRCN increased by 8.6% compared to radiologist assessments. The subgroup analysis conducted on adipose tissue radiomics features further demonstrated their supplementary value in determining the malignancy of pulmonary nodules.
The DLRCN demonstrated good performance in predicting the malignancy of pulmonary nodules, which was comparable to radiologist assessments. The adipose tissue radiomics features have notably enhanced the performance of DLRCN.