{"title":"Analysis of Tandem Repeats in Short-Read Sequencing Data: From Genotyping Known Pathogenic Repeats to Discovering Novel Expansions","authors":"Andreas Halman, Andrew Lonsdale, Alicia Oshlack","doi":"10.1002/cpz1.70010","DOIUrl":null,"url":null,"abstract":"<p>Short tandem repeats (STRs) and variable-number tandem repeats (VNTRs) are repetitive genomic sequences seen widely throughout the genome. These repeat expansions are currently known to cause ∼60 diseases, with expansions in new loci linked to rare diseases continuing to be discovered. Genome sequencing is an important tool for detecting disease-causing variants and several computational tools have been developed to analyze tandem repeats from genomic data, enabling the genotyping and the identification of expanded alleles. However, guidelines for conducting the analysis of these repeats and, more importantly, for assessing the findings are lacking. Understanding the tools and their technical limitations is important for accurately interpreting the results. This article provides detailed, step-by-step instructions for three key use cases in STR analysis from short-read genome sequencing data, which are also applicable to smaller VNTRs. First, it demonstrates an approach for genotyping known pathogenic loci and the identification of clinically significant expansions. Second, we offer guidance on defining tandem repeat loci and conducting genome-wide genotyping studies, which is also applicable to diploid organisms other than humans. Third, instructions are provided on how to find novel expansions at loci not previously known to be associated with disease, aiding in the discovery of new pathogenic loci. Moreover, we introduce the use of newly-developed helper tools that enable a complete and streamlined tandem repeat analysis protocol by addressing the gaps in current methods. All three protocols are compatible with human hg19, hg38, and the latest telomere-to-telomere (hs1) reference genomes. Additionally, this protocol provides an overview and discussion on how to interpret genotyping results. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Genotyping known pathogenic tandem repeat loci</p><p><b>Alternate Protocol</b>: Genotyping known pathogenic tandem repeat loci with STRipy</p><p><b>Support Protocol 1</b>: Installation of tools and ExpansionHunter catalog modification</p><p><b>Basic Protocol 2</b>: Performing genome-wide genotyping of tandem repeats</p><p><b>Basic Protocol 3</b>: Discovering de novo tandem repeat expansions</p><p><b>Support Protocol 2</b>: Compiling ExpansionHunter Denovo from source code and generating STR profiles</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"4 11","pages":""},"PeriodicalIF":2.2000,"publicationDate":"2024-11-05","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.70010","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpz1.70010","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Abstract
Short tandem repeats (STRs) and variable-number tandem repeats (VNTRs) are repetitive genomic sequences seen widely throughout the genome. These repeat expansions are currently known to cause ∼60 diseases, with expansions in new loci linked to rare diseases continuing to be discovered. Genome sequencing is an important tool for detecting disease-causing variants and several computational tools have been developed to analyze tandem repeats from genomic data, enabling the genotyping and the identification of expanded alleles. However, guidelines for conducting the analysis of these repeats and, more importantly, for assessing the findings are lacking. Understanding the tools and their technical limitations is important for accurately interpreting the results. This article provides detailed, step-by-step instructions for three key use cases in STR analysis from short-read genome sequencing data, which are also applicable to smaller VNTRs. First, it demonstrates an approach for genotyping known pathogenic loci and the identification of clinically significant expansions. Second, we offer guidance on defining tandem repeat loci and conducting genome-wide genotyping studies, which is also applicable to diploid organisms other than humans. Third, instructions are provided on how to find novel expansions at loci not previously known to be associated with disease, aiding in the discovery of new pathogenic loci. Moreover, we introduce the use of newly-developed helper tools that enable a complete and streamlined tandem repeat analysis protocol by addressing the gaps in current methods. All three protocols are compatible with human hg19, hg38, and the latest telomere-to-telomere (hs1) reference genomes. Additionally, this protocol provides an overview and discussion on how to interpret genotyping results. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1: Genotyping known pathogenic tandem repeat loci
Alternate Protocol: Genotyping known pathogenic tandem repeat loci with STRipy
Support Protocol 1: Installation of tools and ExpansionHunter catalog modification
Basic Protocol 2: Performing genome-wide genotyping of tandem repeats
Basic Protocol 3: Discovering de novo tandem repeat expansions
Support Protocol 2: Compiling ExpansionHunter Denovo from source code and generating STR profiles

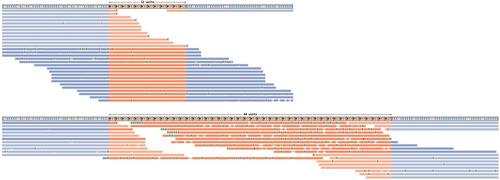
短读数测序数据中的串联重复序列分析:从已知致病性重复序列的基因分型到发现新的扩展。
短串联重复序列(STR)和变数串联重复序列(VNTR)是广泛存在于整个基因组中的重复基因组序列。目前已知这些重复序列的扩展可导致 60 种疾病,而且与罕见疾病相关的新基因位点的扩展仍在不断被发现。基因组测序是检测致病变异的重要工具,目前已开发出几种计算工具来分析基因组数据中的串联重复序列,从而进行基因分型和鉴定扩增等位基因。然而,目前还缺乏对这些重复序列进行分析的指导原则,更重要的是缺乏对分析结果进行评估的指导原则。了解这些工具及其技术局限性对于准确解释结果非常重要。本文为从短读程基因组测序数据中分析 STR 的三个关键用例提供了详细的分步说明,这也适用于较小的 VNTR。首先,它展示了一种对已知致病基因位点进行基因分型和识别具有临床意义的扩增的方法。其次,我们提供了定义串联重复位点和进行全基因组基因分型研究的指导,这也适用于人类以外的二倍体生物。第三,指导如何在以前不知道与疾病相关的位点上发现新的扩增,从而帮助发现新的致病位点。此外,我们还介绍了新开发的辅助工具的使用方法,通过解决当前方法中的不足,实现了完整、简化的串联重复分析方案。所有三个方案都与人类 hg19、hg38 和最新的端粒到端粒(hs1)参考基因组兼容。此外,该方案还概述并讨论了如何解释基因分型结果。© 2024 作者。当前协议》由 Wiley Periodicals LLC 出版。基本方案 1:已知致病性串联重复位点基因分型备用方案:支持协议 1:安装工具和修改 ExpansionHunter 目录 基本协议 2:对串联重复序列进行全基因组范围的基因分型 基本协议 3:发现新的串联重复序列扩增 支持协议 2:从源代码编译 ExpansionHunter Denovo 并生成 STR 图谱。
本文章由计算机程序翻译,如有差异,请以英文原文为准。



