{"title":"Isolation of Small Extracellular Vesicles (sEVs) from the Apoplastic Wash Fluid of Nicotiana benthamiana Leaves","authors":"Mahmoud K. Eldahshoury, Konstantina Katsarou, Joshua T. Farley, Kriton Kalantidis, Carine de Marcos Lousa","doi":"10.1002/cpz1.70026","DOIUrl":null,"url":null,"abstract":"<p>Extracellular vesicles (EVs) are small membranous vesicles secreted by cells into their surrounding extracellular environment. Similar to mammalian EVs, plant EVs have emerged as essential mediators of intercellular communication in plants that facilitate the transfer of biological material between cells. They also play essential roles in diverse physiological processes including stress responses, developmental regulation, and defense mechanisms against pathogens. In addition, plant EVs have demonstrated promising health benefits as well as potential therapeutic effects in mammalian health. Despite the plethora of potential applications using plant EVs, their isolation and characterization remains challenging. In contrast to mammalian EVs, which benefit from more standardized isolation protocols, methods for isolating plant EVs can vary depending on the starting material used, resulting in diverse levels of purity and composition. Additionally, the field suffers from the lack of plant EV markers. Nevertheless, three main EV subclasses have been described from leaf apoplasts: tetraspanin 8 positive (TET8), penetration-1-positive (PEN1), and EXPO vesicles derived from exocyst-positive organelles (EXPO). Here, we present an optimized protocol for the isolation and enrichment of small EVs (sEVs; <200 nm) from the apoplastic fluid from <i>Nicotiana benthamiana</i> leaves by ultracentrifugation. We analyze the preparation through transmitted electron microscopy (TEM), nanoparticle tracking analysis (NTA), and western blotting. We believe this method will establish a basic protocol for the isolation of EVs from <i>N. benthamiana</i> leaves, and we discuss technical considerations to be evaluated by each researcher working towards improving their plant sEV preparations. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol</b>: Isolation and enrichment of small extracellular vesicles (sEVs) from the apoplastic fluid of <i>Nicotiana benthamiana</i> leaves</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"4 11","pages":""},"PeriodicalIF":2.2000,"publicationDate":"2024-11-05","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.70026","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpz1.70026","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Abstract
Extracellular vesicles (EVs) are small membranous vesicles secreted by cells into their surrounding extracellular environment. Similar to mammalian EVs, plant EVs have emerged as essential mediators of intercellular communication in plants that facilitate the transfer of biological material between cells. They also play essential roles in diverse physiological processes including stress responses, developmental regulation, and defense mechanisms against pathogens. In addition, plant EVs have demonstrated promising health benefits as well as potential therapeutic effects in mammalian health. Despite the plethora of potential applications using plant EVs, their isolation and characterization remains challenging. In contrast to mammalian EVs, which benefit from more standardized isolation protocols, methods for isolating plant EVs can vary depending on the starting material used, resulting in diverse levels of purity and composition. Additionally, the field suffers from the lack of plant EV markers. Nevertheless, three main EV subclasses have been described from leaf apoplasts: tetraspanin 8 positive (TET8), penetration-1-positive (PEN1), and EXPO vesicles derived from exocyst-positive organelles (EXPO). Here, we present an optimized protocol for the isolation and enrichment of small EVs (sEVs; <200 nm) from the apoplastic fluid from Nicotiana benthamiana leaves by ultracentrifugation. We analyze the preparation through transmitted electron microscopy (TEM), nanoparticle tracking analysis (NTA), and western blotting. We believe this method will establish a basic protocol for the isolation of EVs from N. benthamiana leaves, and we discuss technical considerations to be evaluated by each researcher working towards improving their plant sEV preparations. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.
Basic Protocol: Isolation and enrichment of small extracellular vesicles (sEVs) from the apoplastic fluid of Nicotiana benthamiana leaves

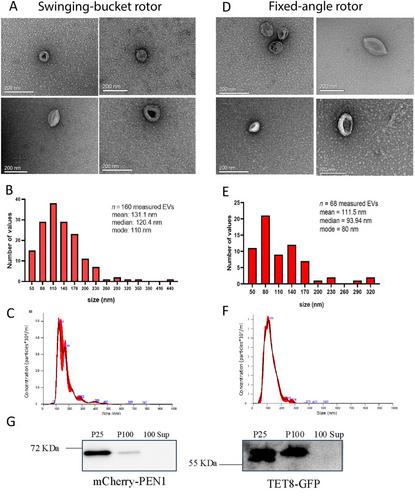
从烟草叶的凋落洗液中分离出小细胞外囊泡 (sEVs)
细胞外囊泡(EVs)是细胞分泌到周围细胞外环境中的小膜囊泡。与哺乳动物的胞外囊泡类似,植物的胞外囊泡已成为植物细胞间通信的重要媒介,可促进生物材料在细胞间的转移。它们在多种生理过程中也发挥着重要作用,包括应激反应、发育调控和病原体防御机制。此外,植物 EVs 还对哺乳动物的健康具有良好的保健作用和潜在的治疗效果。尽管植物 EVs 具有大量潜在应用,但其分离和表征仍然具有挑战性。与哺乳动物的 EVs 不同,植物 EVs 的分离方法会因使用的起始材料而异,从而导致纯度和成分的不同。此外,该领域还缺乏植物 EV 标记。不过,从叶片细胞质中已发现三种主要的 EV 亚类:四泛蛋白 8 阳性(TET8)、渗透-1 阳性(PEN1)和源自外囊阳性细胞器的 EXPO 囊泡。在这里,我们介绍了一种分离和富集小EVs(sEVs)的优化方案;
本文章由计算机程序翻译,如有差异,请以英文原文为准。



