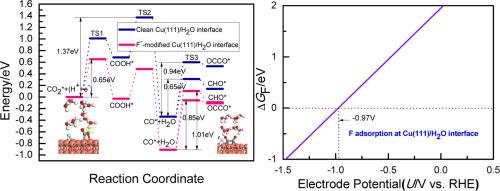
A
F−-modified Cu(111) electrocatalysts exhibited an ultrahigh Faradaic efficiency and product selectivity towards CO
2 electroreduction into C
2 products. Thus, CO
2 electroreduction mechanisms towards C
2H
4 and C
2H
5OH products at
F−-modified Cu(111)/H
2O interface based on DFT calculations are studied in this work, by which the influencing mechanism of specifically adsorbed
F− on electroreduction activity, product selectivity and a alternative C
![]()
C coupling mechanism can be revealed. Our present studies indicate that the presence of
F− can notably enhance CO
2 electroreduction activity towards key intermediate CO because of significantly changed CO
2 adsorption configuration and weakening effect of
F− on COOH adsorption strength. CO dimerization into dimer OCCO through Langmuir-Hinshelwood mechanism is more favorable than CHO formation in the presence of the
F−. Thus, it can be concluded that C
![]()
C coupling reaction occurs via CO dimerization. More CO adsorption strength because of modification of
F− can explain easier occurrence of C
![]()
C coupling reaction, thus leading to change of product selectivity. The parallel OCCOH and OCCHO pathways are proposed for CO
2 electroreduction into C
2H
4 and C
2H
5OH products at
F−-modified Cu(111)/H
2O interface, in which CO dimerization is regarded as the rate-determining step. Ou present studies can unveil CO
2 electroreduction mechanisms and significant role of the specifically adsorbed
F− at Cu/electrolyte interface for promoting CO
2 electroreduction activity and improving product selectivity towards C
2 products.