{"title":"Analyzing Extracellular Vesicle-associated DNA Using Transmission Electron Microscopy at the Single EV-level","authors":"Thupten Tsering, Amélie Nadeau, Janusz Rak, Julia V. Burnier","doi":"10.1002/cpz1.70047","DOIUrl":null,"url":null,"abstract":"<p>Extracellular vesicles (EVs) play an important role in cell-cell communication, carrying bioactive molecules including DNA. EV-associated DNA (EV-DNA) has created enormous interest in the field of biomarkers, particularly related to liquid biopsy. However, its analysis is challenging due to the nanoscale structure of EVs, the low abundance of EV-DNA, and surrounding biogenetic debate. Therefore, novel protocols to enhance the accurate detection of EV-DNA are essential to study its role in normal physiology and disease states. Here, we provide two protocols for confirming the presence of EV-DNA from biological samples. In the first protocol, ultrathin sectioning of EVs is combined with immunogold labeling to detect the presence of double-stranded (ds) DNA within the EV lumen using transmission electron microscopy (TEM). In the second protocol, whole-mount EV immunogold labeling allows detailed morphological analysis of EVs and their surface-associated DNA. Using TEM imaging, we have demonstrated that cancer-cell-derived individual EVs exhibit simultaneous positivity for dsDNA and the EV surface protein tetraspanin 9. We believe that this method can be used to label any proteins of interest inside as well as on the surface of EVs. This can aid in the characterization of single EVs and in the identification and verification of EV-associated biomarkers. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: EV isolation from cell-culture-conditioned medium, EV embedding, ultrathin sectioning, labeling, and imaging</p><p><b>Basic Protocol 2</b>: Whole-mount immunolabeling of EV-DNA</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"4 11","pages":""},"PeriodicalIF":2.2000,"publicationDate":"2024-11-08","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.70047","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpz1.70047","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Abstract
Extracellular vesicles (EVs) play an important role in cell-cell communication, carrying bioactive molecules including DNA. EV-associated DNA (EV-DNA) has created enormous interest in the field of biomarkers, particularly related to liquid biopsy. However, its analysis is challenging due to the nanoscale structure of EVs, the low abundance of EV-DNA, and surrounding biogenetic debate. Therefore, novel protocols to enhance the accurate detection of EV-DNA are essential to study its role in normal physiology and disease states. Here, we provide two protocols for confirming the presence of EV-DNA from biological samples. In the first protocol, ultrathin sectioning of EVs is combined with immunogold labeling to detect the presence of double-stranded (ds) DNA within the EV lumen using transmission electron microscopy (TEM). In the second protocol, whole-mount EV immunogold labeling allows detailed morphological analysis of EVs and their surface-associated DNA. Using TEM imaging, we have demonstrated that cancer-cell-derived individual EVs exhibit simultaneous positivity for dsDNA and the EV surface protein tetraspanin 9. We believe that this method can be used to label any proteins of interest inside as well as on the surface of EVs. This can aid in the characterization of single EVs and in the identification and verification of EV-associated biomarkers. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.
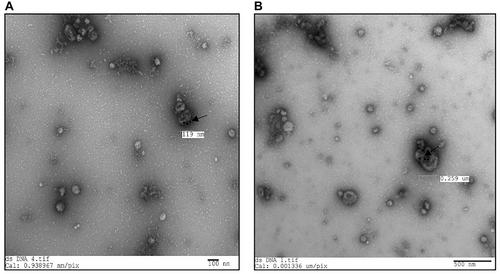
Basic Protocol 1: EV isolation from cell-culture-conditioned medium, EV embedding, ultrathin sectioning, labeling, and imaging
Basic Protocol 2: Whole-mount immunolabeling of EV-DNA

利用透射电子显微镜在单个细胞外囊泡水平分析细胞外囊泡相关 DNA。
细胞外囊泡(EVs)在细胞与细胞之间的交流中发挥着重要作用,携带着包括 DNA 在内的生物活性分子。EV相关DNA(EV-DNA)在生物标记领域引起了极大的兴趣,尤其是与液体活检相关的领域。然而,由于 EVs 的纳米级结构、EV-DNA 的低丰度以及围绕其生物遗传学的争论,对其进行分析具有挑战性。因此,要研究 EV-DNA 在正常生理和疾病状态中的作用,就必须采用新的方案来提高 EV-DNA 的准确检测率。在此,我们提供了两种从生物样本中确认 EV-DNA 存在的方案。在第一种方案中,EV 的超薄切片与免疫金标记相结合,利用透射电子显微镜(TEM)检测 EV 管腔中是否存在双链 (ds) DNA。在第二种方案中,通过对整装 EV 进行免疫金标记,可以对 EV 及其表面相关 DNA 进行详细的形态学分析。通过 TEM 成像,我们证明了癌细胞衍生的单个 EV 同时表现出 dsDNA 和 EV 表面蛋白 tetraspanin 9 阳性。我们相信,这种方法可用于标记 EV 内部和表面的任何相关蛋白质。这有助于单个 EV 的表征以及 EV 相关生物标记物的鉴定和验证。© 2024 作者。当前协议》由 Wiley Periodicals LLC 出版。基本方案 1:从细胞培养调节培养基中分离 EV、EV 包埋、超薄切片、标记和成像 基本方案 2:EV-DNA 的整装免疫标记。
本文章由计算机程序翻译,如有差异,请以英文原文为准。



