Growth retardation and adult height in pediatric patients with chronic-phase chronic myeloid leukemia treated with tyrosine kinase inhibitors
Haruko Shima, Chikako Tono, Akihiko Tanizawa, Masaki Ito, Akihiro Watanabe, Yuki Yuza, Kazuko Hamamoto, Hideki Muramatsu, Masahiko Okada, Shoji Saito, Hiroaki Goto, Masaru Imamura, Akiko M. Saito, Souichi Adachi, Eiichi Ishii, Hiroyuki Shimada
求助PDF
{"title":"Growth retardation and adult height in pediatric patients with chronic-phase chronic myeloid leukemia treated with tyrosine kinase inhibitors","authors":"Haruko Shima, Chikako Tono, Akihiko Tanizawa, Masaki Ito, Akihiro Watanabe, Yuki Yuza, Kazuko Hamamoto, Hideki Muramatsu, Masahiko Okada, Shoji Saito, Hiroaki Goto, Masaru Imamura, Akiko M. Saito, Souichi Adachi, Eiichi Ishii, Hiroyuki Shimada","doi":"10.1038/s41375-024-02488-0","DOIUrl":null,"url":null,"abstract":"","PeriodicalId":18109,"journal":{"name":"Leukemia","volume":"39 2","pages":"508-511"},"PeriodicalIF":13.4000,"publicationDate":"2024-12-12","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Leukemia","FirstCategoryId":"3","ListUrlMain":"https://www.nature.com/articles/s41375-024-02488-0","RegionNum":1,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"HEMATOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
酪氨酸激酶抑制剂治疗慢性粒细胞白血病儿童患者的生长迟缓和成人身高
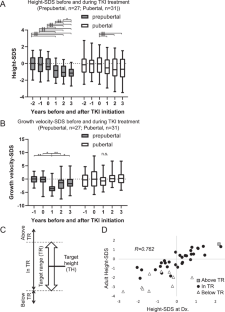
虽然酪氨酸激酶抑制剂(TKIs)可以显著改善慢性髓系白血病(CML)的预后,但长期暴露于TKI仍然是一个主要问题,特别是在儿童中。治疗TKIs儿童的一个关键挑战是生长迟缓[1,2,3,4]。最近,据报道,第一代TKI伊马替尼和第二代TKI达沙替尼都能影响费城染色体阳性急性淋巴细胞白血病患者的生长。我们之前报道过伊马替尼治疗CML儿童的生长迟缓,特别是那些在青春期前治疗的儿童。尽管患者在青春期经常经历追赶生长,但这种追赶的程度以及TKI暴露对成人身高的长期影响仍然知之甚少。我们前瞻性地收集了日本儿童白血病和淋巴瘤研究组(JPLSG) CML-08研究中被诊断为慢性期CML的儿童的数据。身高数据每年收集一次,从诊断前两年开始。成人高度定义为高度增长速度为1 cm/年时所测得的最大高度。身高标准偏差评分(SDS)采用年龄和性别调整的日本标准[6]进行调整,成人身高标准偏差评分采用17岁零6个月的数据。青春期定义为男性睾丸≥3ml,女性乳房≥Tanner 2。定义峰高速度为生长突增时的高速度增量,根据生长曲线[7]计算峰高速度下的年龄。生长突增前的TKI暴露时间定义为TKI暴露至峰高速度的持续时间。收集亲代身高数据,预测成虫身高和范围。目标身高(TH)和目标身高的95%置信区间(TR)的计算公式如下(cm):男性,TH =(母亲身高+父亲身高+ 13)/2,TR = TH±9;女性,(母亲的身高+父亲的身高−13)/ 2,TR = TH±8[8]。
本文章由计算机程序翻译,如有差异,请以英文原文为准。