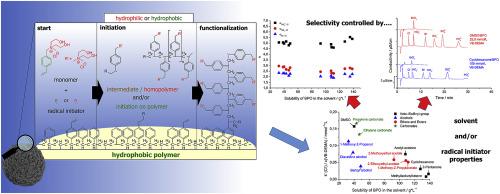
A previously published radical graft-functionalization method for the synthesis of high performance anion exchangers was further investigated to control the capacity and selectivity of the exchangers. Using a hydrophobic radical initiator instead of a hydrophilic one diminished the influence of rivaling homopolymerization of monomer during the functionalization step. Instead of only generating monomer radicals in free solution the radicals are ideally generated on top of the PS/DVB surface. However, in both cases the selectivity factors of polarizable anions bromide and nitrate in relation to chloride increased strongly with increasing capacity of the exchanger. Higher exchanger capacities could lead to coelution of bromide and/or nitrate with other analytes such as sulfate or phosphate when using the eluent as proposed in this work. By variation of the organic solvent used for functionalization it was possible to remove both the rivaling homopolymerization and the strong influence of the capacity on the selectivity. With increasing solubility of the hydrophobic radical initiator in the organic solvent the influence of the homopolymerization and the influence on the selectivity factor of bromide and nitrate decreased. Additionally, a change of bromate selectivity factor could be observed. The bromate signal is shifted closer towards the chloride signal. However, with increasing solubility of the radical initiator in the organic solvent the observed capacity of the exchangers decreases linearly, resulting in higher amounts of monomer needed for functionalization.