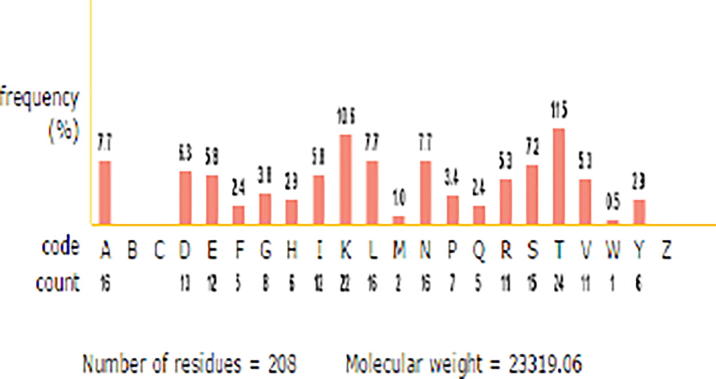
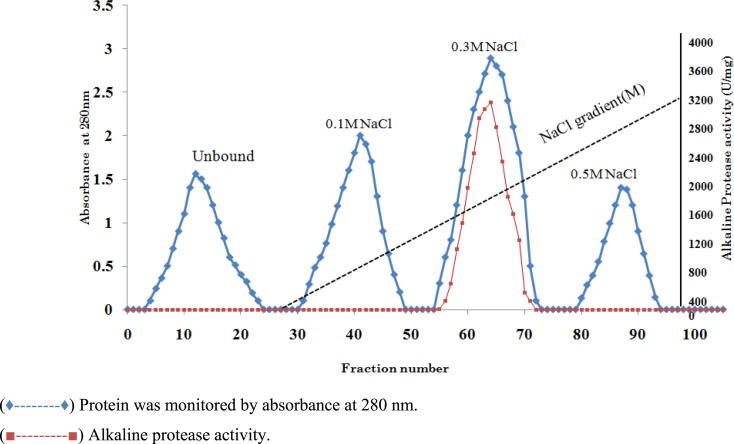
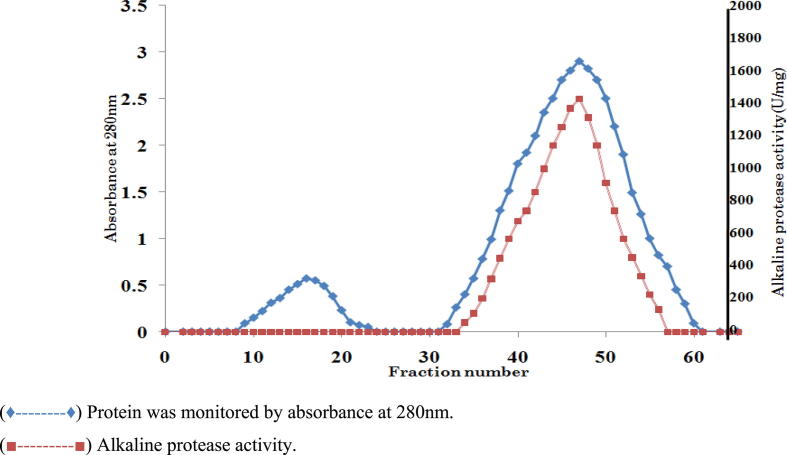
Proteases are the hydrolytic enzymes which hydrolyzes peptide bond between proteins with paramount applications in pharmaceutical and industrial sector. Therefore production of proteases with efficient characteristics of biotechnological interest from novel strain is significant. Hence, in this study, an alkaline serine protease produced by Bacillus cereus strain S8 (MTCC NO 11901) was purified and characterized. The alkaline protease was purified by ammonium sulfate precipitation (50%), ion exchange (DEAE-Cellulose) and gel filtration (Sephadex G-100) chromatographic techniques. As a result of this purification, a protein with specific activity of 300U/mg protein was obtained with purification fold 17.04 and recovery percentage of 34.6%. The molecular weight of the purified protease was determined using SDS-PAGE under non-reducing (71 kDa) and reducing conditions (35 kDa and 22 kDa). Zymogram analysis revealed that proteolytic activity was only associated with 22 kDa. These results indicate that existence of the enzyme as dimer in its native state. The molecular weight of the protease (22 kDa) was also determined by gel filtration (Sephadex G-200) chromatography and it was calculated as 21.8 kDa. The optimum activity of the protease was observed at pH 10.0 and temperature 70 °C with great stability towards pH and temperature with casein as a specific substrate. The enzyme was completely inhibited by PMSF and TLCK indicating that it is a serine protease of trypsin type. The enzyme exhibits a great stability towards organic solvents, oxidizing and bleaching agents and it is negatively influenced by Li2+ and Co2+ metal ions. The purified protein was further characterized by Matrix Assisted Laser Desorption Ionization/Mass Spectroscopy (MALDI/MS) analysis which reveals that total number of amino acids is 208 with isoelectric point 9.52.