In many cellular processes including cell division, the synergistic dynamics of actin filaments and microtubules play vital roles. However, the regulatory mechanisms of these synergistic dynamics are not fully understood. Proteins such as formins are involved in actin filament–microtubule interactions and Arabidopsis thaliana formin 14 (AtFH14) may function as a crosslinker between actin filaments and microtubules in cell division, but the molecular mechanism underlying such crosslinking remains unclear.
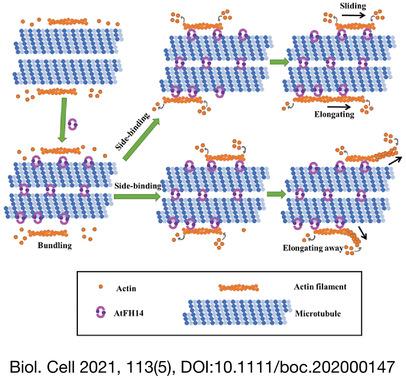
Without microtubules, formin homology (FH) 1/FH2 of AtFH14 nucleated actin polymerisation from actin monomers and capped the barbed end of actin filaments. However, in the presence of microtubules, quantitative analysis showed that the binding affinity of AtFH14 FH1FH2 to microtubules was higher than that to actin filaments. Moreover, microtubule-bound AtFH14 FH1FH2 neither nucleated actin polymerisation nor inhibited barbed end elongation. In contrast, tubulin did not affect AtFH14 FH1FH2 to nucleate actin polymerisation and inhibit barbed end elongation. Nevertheless, microtubule-bound AtFH14 FH1FH2 bound actin filaments and the bound actin filaments slid and elongated along the microtubules or elongated away from the microtubules, which induced bundling or crosslinking of actin filaments and microtubules. Pharmacological analyses indicated that AtFH14 FH1FH2 promoted crosslinking of actin filaments and microtubules in vivo. Additionally, co-sedimentation and fluorescent dye-labelling experiments of AtFH14 FH2-truncated proteins in vitro revealed the essential motifs of bundling actin filaments or microtubules, which were 63–92 aa and 42–62 aa in the AtFH14 FH2 N-terminal, respectively, and 42–62 aa was the essential motif to crosslink actin filaments and microtubules.
Our results aid in explaining how AtFH14 functions as a crosslinker between actin filaments and microtubules to regulate their dynamics via different manners during cell division. They also facilitate further understanding of the molecular mechanisms of the interactions between actin filaments and microtubules.