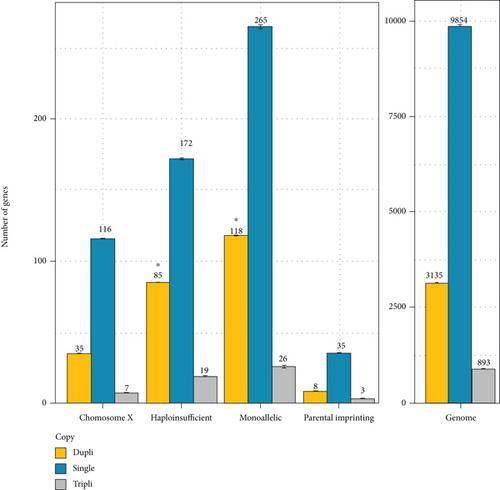
Gene dosage is an important issue both in cell and evolutionary biology. Most genes are present in two copies or alleles in diploid eukariotic cells. The most outstanding exception is monoallelic gene expression (MA) that concerns genes localized on the X chromosome or in regions undergoing parental imprinting in eutherians, and many other genes scattered throughout the genome. In diploids, haploinsufficiency (HI) implies that a single functional copy of a gene in a diploid organism is insufficient to ensure a normal biological function. One of the most important mechanisms ensuring functional innovation during evolution is whole genome duplication (WGD). In addition to the two WGDs that have occurred in vertebrate genomes, the teleost genomes underwent an additional WGD, after their divergence from tetrapods. In the present work, we have studied on 57 teleost species whether the orthologs of human MA or HI genes remain more frequently in duplicates or returned more frequently in singleton than the rest of the genome. Our results show that the teleost orthologs of HI human genes remained more frequently in duplicate than the rest of the genome in all of the teleost species studied. No signal was observed for the orthologs of genes mapping to the human X chromosome or subjected to parental imprinting. Surprisingly, the teleost orthologs of the other human MA genes remained in duplicate more frequently than the rest of the genome for most teleost species. These results suggest that the teleost orthologs of MA and HI human genes also undergo selective pressures either related to absolute protein amounts and/or of dosage balance issues. However, these constraints seem to be different for MA genes in teleost in comparison with human genomes.