Targeting the TWEAK–Fn14 pathway prevents dysfunction in cardiac calcium handling after acute kidney injury
Jonay Poveda, Laura González-Lafuente, Sara Vázquez-Sánchez, Elisa Mercado-García, Elena Rodríguez-Sánchez, Inés García-Consuegra, Ana Belén Sanz, Julián Segura, María Fernández-Velasco, Fernando Liaño, Luis M Ruilope, Gema Ruiz-Hurtado
下载PDF
{"title":"Targeting the TWEAK–Fn14 pathway prevents dysfunction in cardiac calcium handling after acute kidney injury","authors":"Jonay Poveda, Laura González-Lafuente, Sara Vázquez-Sánchez, Elisa Mercado-García, Elena Rodríguez-Sánchez, Inés García-Consuegra, Ana Belén Sanz, Julián Segura, María Fernández-Velasco, Fernando Liaño, Luis M Ruilope, Gema Ruiz-Hurtado","doi":"10.1002/path.6200","DOIUrl":null,"url":null,"abstract":"<p>Heart and kidney have a closely interrelated pathophysiology. Acute kidney injury (AKI) is associated with significantly increased rates of cardiovascular events, a relationship defined as cardiorenal syndrome type 3 (CRS3). The underlying mechanisms that trigger heart disease remain, however, unknown, particularly concerning the clinical impact of AKI on cardiac outcomes and overall mortality. Tumour necrosis factor-like weak inducer of apoptosis (TWEAK) and its receptor fibroblast growth factor-inducible 14 (Fn14) are independently involved in the pathogenesis of both heart and kidney failure, and recent studies have proposed TWEAK as a possible therapeutic target; however, its specific role in cardiac damage associated with CRS3 remains to be clarified. Firstly, we demonstrated in a retrospective longitudinal clinical study that soluble TWEAK plasma levels were a predictive biomarker of mortality in patients with AKI. Furthermore, the exogenous application of TWEAK to native ventricular cardiomyocytes induced relevant calcium (Ca<sup>2+</sup>) handling alterations. Next, we investigated the role of the TWEAK–Fn14 axis in cardiomyocyte function following renal ischaemia–reperfusion (I/R) injury in mice. We observed that TWEAK–Fn14 signalling was activated in the hearts of AKI mice. Mice also showed significantly altered intra-cardiomyocyte Ca<sup>2+</sup> handling and arrhythmogenic Ca<sup>2+</sup> events through an impairment in sarcoplasmic reticulum Ca<sup>2+</sup>-adenosine triphosphatase 2a pump (SERCA<sub>2a</sub>) and ryanodine receptor (RyR<sub>2</sub>) function. Administration of anti-TWEAK antibody after reperfusion significantly improved alterations in Ca<sup>2+</sup> cycling and arrhythmogenic events and prevented SERCA<sub>2a</sub> and RyR<sub>2</sub> modifications. In conclusion, this study establishes the relevance of the TWEAK–Fn14 pathway in cardiac dysfunction linked to CRS3, both as a predictor of mortality in patients with AKI and as a Ca<sup>2+</sup> mishandling inducer in cardiomyocytes, and highlights the cardioprotective benefits of TWEAK targeting in CRS3. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"261 4","pages":"427-441"},"PeriodicalIF":5.2000,"publicationDate":"2023-09-30","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://pathsocjournals.onlinelibrary.wiley.com/doi/epdf/10.1002/path.6200","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6200","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
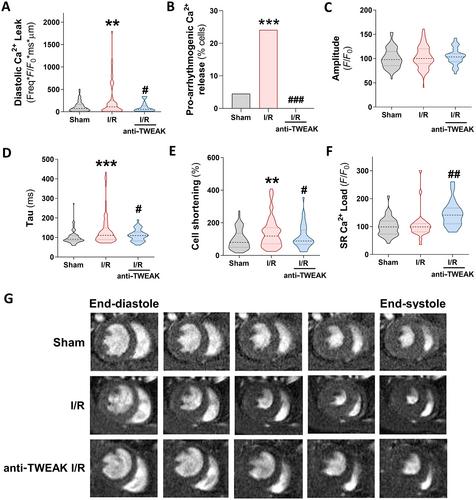
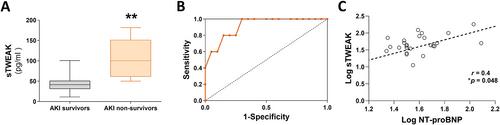
Heart and kidney have a closely interrelated pathophysiology. Acute kidney injury (AKI) is associated with significantly increased rates of cardiovascular events, a relationship defined as cardiorenal syndrome type 3 (CRS3). The underlying mechanisms that trigger heart disease remain, however, unknown, particularly concerning the clinical impact of AKI on cardiac outcomes and overall mortality. Tumour necrosis factor-like weak inducer of apoptosis (TWEAK) and its receptor fibroblast growth factor-inducible 14 (Fn14) are independently involved in the pathogenesis of both heart and kidney failure, and recent studies have proposed TWEAK as a possible therapeutic target; however, its specific role in cardiac damage associated with CRS3 remains to be clarified. Firstly, we demonstrated in a retrospective longitudinal clinical study that soluble TWEAK plasma levels were a predictive biomarker of mortality in patients with AKI. Furthermore, the exogenous application of TWEAK to native ventricular cardiomyocytes induced relevant calcium (Ca2+ ) handling alterations. Next, we investigated the role of the TWEAK–Fn14 axis in cardiomyocyte function following renal ischaemia–reperfusion (I/R) injury in mice. We observed that TWEAK–Fn14 signalling was activated in the hearts of AKI mice. Mice also showed significantly altered intra-cardiomyocyte Ca2+ handling and arrhythmogenic Ca2+ events through an impairment in sarcoplasmic reticulum Ca2+ -adenosine triphosphatase 2a pump (SERCA2a ) and ryanodine receptor (RyR2 ) function. Administration of anti-TWEAK antibody after reperfusion significantly improved alterations in Ca2+ cycling and arrhythmogenic events and prevented SERCA2a and RyR2 modifications. In conclusion, this study establishes the relevance of the TWEAK–Fn14 pathway in cardiac dysfunction linked to CRS3, both as a predictor of mortality in patients with AKI and as a Ca2+ mishandling inducer in cardiomyocytes, and highlights the cardioprotective benefits of TWEAK targeting in CRS3. © 2023 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
靶向TWEAK-Fn14通路可预防急性肾损伤后心脏钙处理功能障碍
心脏和肾脏具有密切相关的病理生理。急性肾损伤(AKI)与心血管事件发生率显著增加相关,这种关系被定义为心肾综合征3型(CRS3)。然而,引发心脏病的潜在机制仍然未知,特别是AKI对心脏结局和总死亡率的临床影响。肿瘤坏死因子样细胞凋亡弱诱导剂(TWEAK)及其受体成纤维细胞生长因子诱导14 (Fn14)独立参与心衰和肾衰竭的发病机制,近期研究提出TWEAK可能作为治疗靶点;然而,其在与CRS3相关的心脏损伤中的具体作用仍有待阐明。首先,我们在一项回顾性纵向临床研究中证明,可溶性TWEAK血浆水平是AKI患者死亡率的预测性生物标志物。此外,外源性应用TWEAK原生心室心肌细胞诱导相关钙(Ca2+)处理改变。接下来,我们研究了TWEAK-Fn14轴在小鼠肾缺血再灌注(I/R)损伤后心肌细胞功能中的作用。我们观察到TWEAK-Fn14信号在AKI小鼠的心脏中被激活。小鼠还通过肌浆网Ca2+-腺苷三磷酸酶2a泵(SERCA2a)和ryanodine受体(RyR2)功能的损害,显着改变了心肌细胞内Ca2+处理和心律失常Ca2+事件。再灌注后给予抗tweak抗体可显著改善Ca2+循环和心律失常事件的改变,并阻止SERCA2a和RyR2修饰。总之,本研究确定了与CRS3相关的心功能障碍中TWEAK - fn14通路的相关性,既可以作为AKI患者死亡率的预测因子,也可以作为心肌细胞中Ca2+处理不当的诱导剂,并强调了针对CRS3的TWEAK的心脏保护益处。©2023作者。《病理学杂志》由约翰·威利出版;儿子有限公司代表大不列颠及爱尔兰病理学会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。