Late secondary glaucoma is an often-severe complication after acute events like anterior segment surgery, trauma and infection. TNF-α is a major mediator that is rapidly upregulated, diffusing also to the retina and causes apoptosis of the ganglion cells and degeneration of their optic nerve axons (mediating steps to glaucomatous damage). Anti-TNF-α antibodies are in animals very effective in protecting the retinal cells and the optic nerve—and might therefore be useful prophylactically against secondary glaucoma in future such patients. Here we evaluate (1) toxicity and (2) efficacy of two TNF-α inhibitors (adalimumab and infliximab), in rabbits by subconjunctival administration.
For drug toxicity, animals with normal, unburned corneas were injected with adalimumab (0.4, 4, or 40 mg), or infliximab (1, 10, or 100 mg). For drug efficacy, other animals were subjected to alkali burn before such injection, or steroids (for control). The rabbits were evaluated clinically with slit lamp and photography, electroretinography, optical coherence tomography, and intraocular pressure manometry. A sub-set of eyes were stained ex vivo after 3 days for retinal cell apoptosis (TUNEL). In other experiments the optic nerves were evaluated by paraphenylenediamine staining after 50 or 90 days. Loss of retinal cells and optic nerve degeneration were quantified.
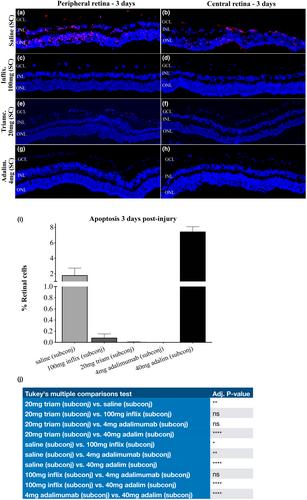
Subconjunctival administration of 0.4 mg or 4.0 mg adalimumab were well tolerated, whereas 40.0 mg was toxic to the retina. 1, 10, or 100 mg infliximab were also well tolerated. Analysis of the optic nerve axons after 50 days confirmed the safety of 4.0 mg adalimumab and of 100 mg infliximab. For efficacy, 4.0 mg adalimumab subconjunctivally in 0.08 mL provided practically full protection against retinal cell apoptosis 3 days following alkali burn, and infliximab 100 mg only slightly less. At 90 days following burn injury, control optic nerves showed about 50% axon loss as compared to 8% in the adalimumab treatment group.
Subconjunctival injection of 4.0 mg adalimumab in rabbits shows no eye toxicity and provides excellent neuroprotection, both short (3 days) and long-term (90 days). Our total. accumulated data from several of our studies, combined with the present paper, suggest that corneal injuries, including surgery, might benefit from routine administration of anti-TNF-α biologics to reduce inflammation and future secondary glaucoma.