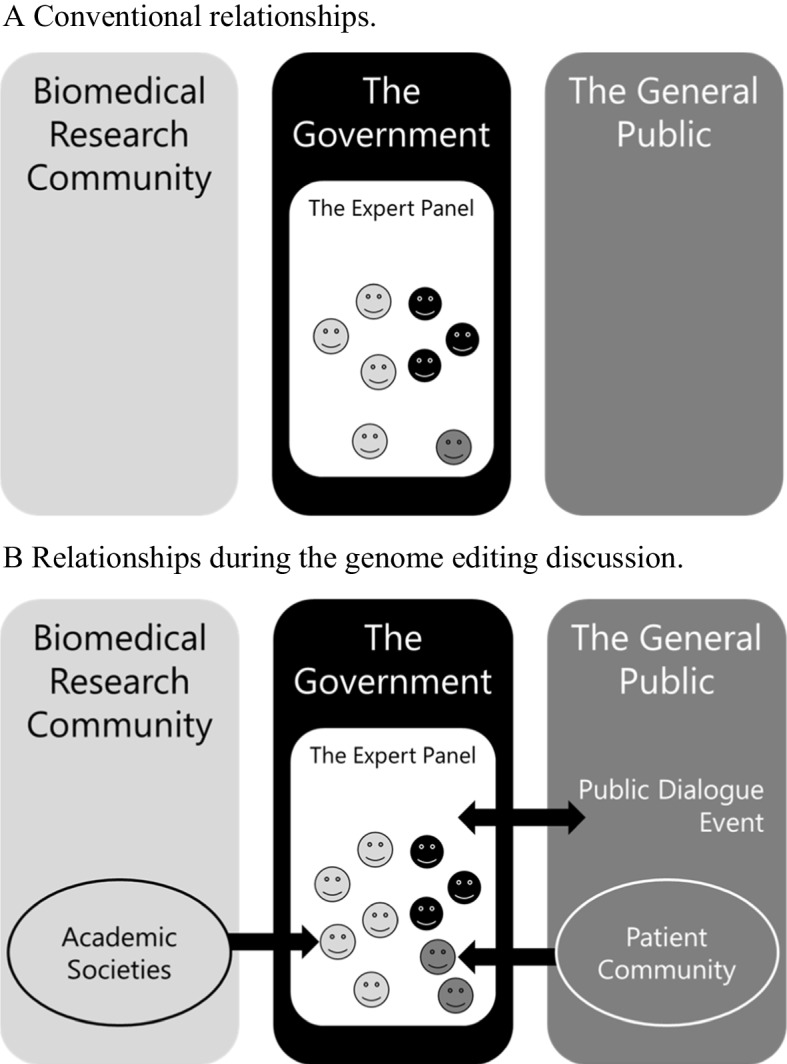
Genome editing is a technology that can accurately and efficiently modify the genome of organisms, including the human genome. Although human genome editing (HGE) has many benefits, it also involves technical risks and ethical, legal, and social issues. Thus, the pros and cons of using this technology have been actively debated since 2015. Notably, the research community has taken an interest in the issue and has discussed it internationally. However, for the governance of HGE, the roles of government agencies and the general public are also important for an effective regulatory system. Here, we examine the roles of the research community, government, and public in the governance of HGE through an analysis of discussions in the Japanese Expert Panel on Bioethics. During the discussion of the research ethics review system, the professionalism of the research community and the pros and cons of state oversight have become issues for debate. Furthermore, through an examination of the overall policy-making process, three stakeholders are clearly involved in the governance of emerging medical technologies in the Expert Panel on Bioethics, a discussion forum established by government agencies. The contrast among these roles provides insight into the positive roles of government agencies and the research community and the conditions under which these roles are played. We also note that there are diverse actors in the public, which may have an impact on their participation. Our results may serve as a guide for countries and organizations to establish governance on emerging medical technologies.