The dysregulation of TGF-β signaling is a crucial pathophysiological process in tumorigenesis and progression. LncRNAs have diverse biological functions and are significant participants in the regulation of tumor signaling pathways. However, the clinical value of lncRNAs related to TGF-β signaling in glioma is currently unclear.
Data on glioma's RNA-seq transcriptome, somatic mutation, DNA methylation data, and clinicopathological information were derived from the CGGA and TCGA databases. A prognostic lncRNA signature was constructed by Cox and LASSO regression analyses. TIMER2.0 database was utilized to deduce immune infiltration characteristics. “ELMER v.2” was used to reconstruct TF-methylation-gene regulatory network. Immunotherapy and chemotherapy response predictions were implemented by the TIDE algorithm and GDSC database, respectively. In vitro and in vivo experiments were conducted to verify the results and clarify the regulatory mechanism of lncRNA.
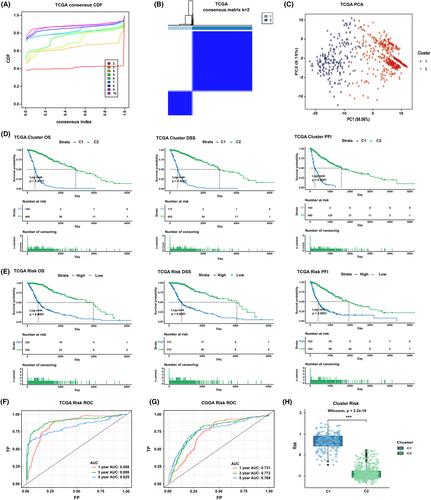
In glioma, a TGF-β signaling-related 15-lncRNA signature was constructed, including AC010173.1, HOXA-AS2, AC074286.1, AL592424.1, DRAIC, HOXC13-AS, AC007938.1, AC010729.1, AC013472.3, AC093895.1, AC131097.4, AL606970.4, HOXC-AS1, AGAP2-AS1, and AC002456.1. This signature proved to be a reliable prognostic tool, with high risk indicating an unfavorable prognosis and being linked to malignant clinicopathological and genomic mutation traits. Risk levels were associated with different immune infiltration landscapes, where high risk was indicative of high levels of macrophage infiltration. In addition, high risk also suggested better immunotherapy and chemotherapy response. cg05987823 was an important methylation site in glioma progression, and AP-1 transcription factor family participated in the regulation of signature lncRNA expression. AGAP2-AS1 knockdown in in vitro and in vivo experiments inhibited the proliferation, migration, and invasion of glioma cells, as well as the growth of glioma, by downregulating the expression levels of NF-κB and ERK 1/2 in the TGF-β signaling pathway.
A prognostic lncRNA signature of TGF-β signaling was established in glioma, which can be used for prognostic judgment, immune infiltration status inference, and immunotherapy response prediction. AGAP2-AS1 plays an important role in glioma progression.