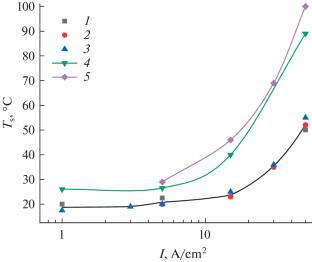
Experimental study of the anodic dissolution of titanium and its alloys over a wide range of current densities, including pulsed currents (up to 100 A/cm2), under controlled hydrodynamic conditions and surface temperature in nitrate and chloride solutions, showed that the process is mediated by electrochemical formation of an anodic oxide film (AOF), which undergoes chemical dissolution. The AOF has a bilayer structure (two barrier films: at the interface with the metal and solution). It is described by PDM-III (Point Defect Model). Under certain conditions, it is possible to achieve a steady state in which the film growth rate is compensated by the rate of its chemical dissolution (during a pulsed treatment). In this case, there is a 100% current efficiency in terms of titanium ionization in the oxidation state of four. Under the conditions of the described experiments, i.e., when using direct current, the rate of the AOF electrochemical formation exceeds that of its chemical dissolution, which leads to a decrease in the current efficiency, which does not exceed 75%. Due to the temperature dependence of the electrical resistance of the barrier film at the interface with the solution, which determines its thickness, the current efficiency increases with an increase in the flow rate of the electrolyte. When the thermokinetic instability (TKI) of the AOF is reached (thermal explosion caused by positive feedback: the rate of electrochemical reaction–surface temperature–the rate of electrochemical reaction), the interaction of electrolyte components with the surface free from the film leads to “anomalous” anodic dissolution of the AOF with a current efficiency exceeding 100%. Regardless of the nature of the electrolyte, the TKI conditions are reached at ~1 A/cm2. It has been shown that the dissolution rate in nitrate solutions for certain pulsed treatment parameters (relative pulse duration of 2, dc = 50%) (and the displacement of cathode tool in electrochemical machining) may exceed the machining rate with direct current of the same density by more than a factor of two.