Patients with systemic autoimmune disease are predisposed to developing sarcopenia associated with their underlying proinflammatory condition and a decrease in motility. How sarcopenic status affects disease progression remains unknown. The present study explored the influence of denervation-induced sarcopenia on immune homeostasis and investigated related biological pathways that might be important for future disease management.
A denervation-induced skeletal muscle loss model was established, and the function of the immune system was evaluated. Immune cell proportions and cytokine levels in peripheral blood and atrophied skeletal muscle were profiled. We examined store-operated Ca2+ entry and mitochondrial function in immune cells. MRL/lpr mice were used to evaluate the influence of denervation-induced sarcopenia on autoimmune progression in lupus nephritis.
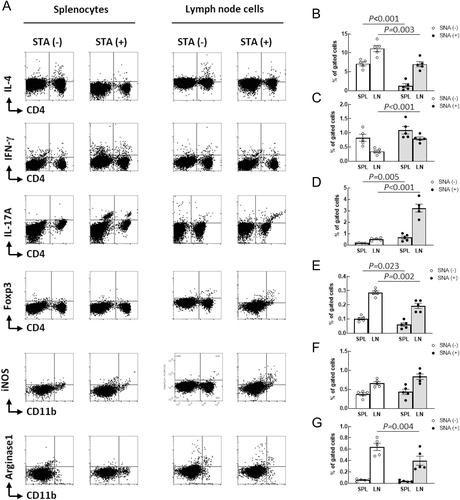
Compared with splenocyte fractions obtained from sham mice, proportions of CD4+ cells, CD8+ cells, CD19+CD20− cells, CD49b+NK46+ cells, CD4+IL-4+ cells, and CD4+FoxP3+ cells were significantly decreased, whereas CD19+CD20+ cells, CD4+IFN-γ+ cells, and CD4+IL17+ cells were markedly increased in sciatic nerve axotomy (SNA) mice. The serum cytokine profile indicated that SNA significantly increased the protein expressions of IL-12p70 (P = 0.014), IL-17A (P = 0.007), and IFN-γ (P < 0.001). In SNA mice, there was an 18.74% decrease in peak Ca2+ influx (P < 0.001) and an 18.22% decrease in initial Ca2+ influx (P < 0.001) rates in peripheral T cells compared with sham mice. Mitochondrial respiration was significantly reduced in peripheral T cells from SNA mice compared with sham mice. SNA substantially promoted disease progression in lupus model mice demonstrated by the results of proteinuria monitoring, serum levels of blood urea nitrogen, and kidney histological scores.
The loss of skeletal muscle impaired mitochondrial respiration and activation in T cells and influenced the balance of T helper cell subsets. Therefore, denervation-induced sarcopenia might promote the progression of autoimmune diseases, such as lupus nephritis, and enhance Th1/Th17 functions. These results suggest that physicians should be aware of the impact of the loss of skeletal muscle on the management of autoimmune disease and that a multidisciplinary approach is required to minimize the overall adverse impact of sarcopenia.