下载PDF
{"title":"Rapid Identification of Protein-Protein Interactions in Plants","authors":"Youjun Zhang, Roberto Natale, Adilson Pereira Domingues Júnior, Mitchell Rey Toleco, Beata Siemiatkowska, Norma Fàbregas, Alisdair R. Fernie","doi":"10.1002/cppb.20099","DOIUrl":null,"url":null,"abstract":"<p>Enzyme-enzyme interactions can be discovered by affinity purification mass spectrometry (AP-MS) under in vivo conditions. Tagged enzymes can either be transiently transformed into plant leaves or stably transformed into plant cells prior to AP-MS. The success of AP-MS depends on the levels and stability of the bait protein, the stability of the protein-protein interactions, and the efficiency of trypsin digestion and recovery of tryptic peptides for MS analysis. Unlike in-gel-digestion AP-MS, in which the gel is cut into pieces for several independent trypsin digestions, we uses a proteomics-based in-solution digestion method to directly digest the proteins on the beads following affinity purification. Thus, a single replicate within an AP-MS experiment constitutes a single sample for LC-MS measurement. In subsequent data analysis, normalized signal intensities can be processed to determine fold-change abundance (FC-A) scores by use of the SAINT algorithm embedded within the CRAPome software. Following analysis of co-sublocalization of “bait” and “prey,” we suggest considering only the protein pairs for which the intensities were more than 2% compared with the bait, corresponding to FC-A values of at least four within-biological replicates, which we recommend as minimum. If the procedure is faithfully followed, experimental assessment of enzyme-enzyme interactions can be carried out in Arabidopsis within 3 weeks (transient expression) or 5 weeks (stable expression). © 2019 The Authors.</p><p><b>Basic Protocol 1</b>: Gene cloning to the destination vectors</p><p><b>Alternate Protocol</b>: In-Fusion or Gibson gene cloning protocol</p><p><b>Basic Protocol 2</b>: Transformation of baits into the plant cell culture or plant leaf</p><p><b>Basic Protocol 3</b>: Affinity purification of protein complexes</p><p><b>Basic Protocol 4</b>: On-bead trypsin/LysC digestion and C18 column peptide desalting and concentration</p><p><b>Basic Protocol 5</b>: Data analysis and quality control</p>","PeriodicalId":10932,"journal":{"name":"Current protocols in plant biology","volume":"4 4","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2019-11-12","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/cppb.20099","citationCount":"19","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols in plant biology","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cppb.20099","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"Agricultural and Biological Sciences","Score":null,"Total":0}
引用次数: 19
引用
批量引用
Abstract
Enzyme-enzyme interactions can be discovered by affinity purification mass spectrometry (AP-MS) under in vivo conditions. Tagged enzymes can either be transiently transformed into plant leaves or stably transformed into plant cells prior to AP-MS. The success of AP-MS depends on the levels and stability of the bait protein, the stability of the protein-protein interactions, and the efficiency of trypsin digestion and recovery of tryptic peptides for MS analysis. Unlike in-gel-digestion AP-MS, in which the gel is cut into pieces for several independent trypsin digestions, we uses a proteomics-based in-solution digestion method to directly digest the proteins on the beads following affinity purification. Thus, a single replicate within an AP-MS experiment constitutes a single sample for LC-MS measurement. In subsequent data analysis, normalized signal intensities can be processed to determine fold-change abundance (FC-A) scores by use of the SAINT algorithm embedded within the CRAPome software. Following analysis of co-sublocalization of “bait” and “prey,” we suggest considering only the protein pairs for which the intensities were more than 2% compared with the bait, corresponding to FC-A values of at least four within-biological replicates, which we recommend as minimum. If the procedure is faithfully followed, experimental assessment of enzyme-enzyme interactions can be carried out in Arabidopsis within 3 weeks (transient expression) or 5 weeks (stable expression). © 2019 The Authors.
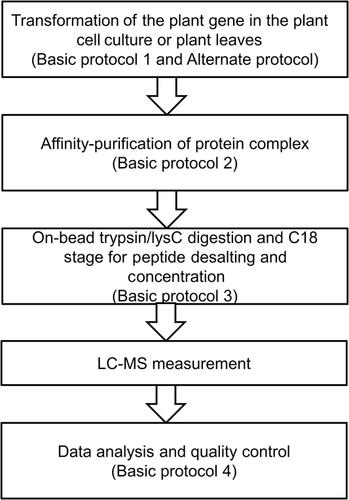
Basic Protocol 1 : Gene cloning to the destination vectors
Alternate Protocol : In-Fusion or Gibson gene cloning protocol
Basic Protocol 2 : Transformation of baits into the plant cell culture or plant leaf
Basic Protocol 3 : Affinity purification of protein complexes
Basic Protocol 4 : On-bead trypsin/LysC digestion and C18 column peptide desalting and concentration
Basic Protocol 5 : Data analysis and quality control
植物中蛋白质-蛋白质相互作用的快速鉴定
酶-酶的相互作用可以通过亲和纯化质谱(AP-MS)在体内条件下发现。在AP-MS之前,标记的酶可以瞬间转化为植物叶片,也可以稳定地转化为植物细胞。AP-MS的成功取决于诱饵蛋白的水平和稳定性,蛋白-蛋白相互作用的稳定性,以及胰蛋白酶消化和用于MS分析的胰蛋白酶肽的回收效率。与凝胶消解AP-MS不同,凝胶被切成几块用于几个独立的胰蛋白酶消解,我们使用基于蛋白质组学的溶液消解方法,在亲和纯化后直接消解珠子上的蛋白质。因此,AP-MS实验中的单个重复构成LC-MS测量的单个样品。在随后的数据分析中,可以使用CRAPome软件中嵌入的SAINT算法对归一化信号强度进行处理,以确定fold-change abundance (FC-A)评分。在对“诱饵”和“猎物”的共亚定位进行分析后,我们建议只考虑与诱饵相比强度大于2%的蛋白质对,对应于至少四个生物重复内的FC-A值,我们建议将其作为最小值。如果忠实地遵循程序,可以在3周(瞬时表达)或5周(稳定表达)内在拟南芥中进行酶-酶相互作用的实验评估。©2019作者。基本方案1:基因克隆到目的载体替代方案:In-Fusion或Gibson基因克隆方案基本方案2:将诱饵转化为植物细胞培养或植物叶片基本方案3:蛋白质复合物的亲和纯化基本方案4:头上胰蛋白酶/LysC消化和C18柱肽脱盐和浓缩基本方案5:数据分析和质量控制
本文章由计算机程序翻译,如有差异,请以英文原文为准。