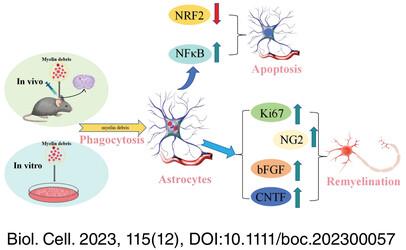
Persistent myelin debris can inhibit axonal regeneration, thereby hindering remyelination. Effective removal of myelin debris is essential to eliminate the interference of myelin debris in oligodendrocyte progenitor cell (OPC) activation, recruitment to demyelinating sites and/or differentiation into mature oligodendrocytes (OLs). In addition to microglia, it has been reported that astrocytic phagocytosis of myelin debris is a feature of early demyelination.
In the present study, astrocytes effectively phagocytized myelin debris in vitro and in vivo. On the 5th day after injecting myelin debris into the brain, astrocytes were enriched in the area injected with myelin debris compared with microglia, and their ability to engulf myelin debris was stronger than that of microglia. When exposed to myelin debris, astrocytes phagocytizing myelin debris triggered self-apoptosis, accompanied by the activation of NF-κB, down-regulation of Nrf2, and the increase of ciliary neurotrophic factor (CNTF) and basic fibroblast growth factor (bFGF). However, the activation of astrocytic NF-κB did not influence the inflammatory cytokines IL-1β, IL-6, and TNF-α, and the anti-inflammatory factor IL-10. The proliferation of astrocytes and mobilization of OPCs in the subventricular zone were elevated on the 5th day after intracerebral injection of myelin debris.
The results suggested that myelin phagocytosis of astrocytes should help improve the microenvironment and promote myelin regeneration by increasing CNTF and bFGF within the central nervous system.
However, the molecular interaction of astrocytes acting as phagocytes remains to be further explored. Therefore, an improvement of astrocytes to phagocytize myelin debris may be a promising treatment measure to prevent demyelination and promote remyelination in MS and other diseases with prominent myelin injury.