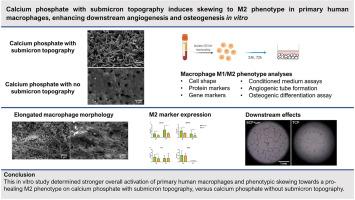
Calcium phosphates with submicron surface features have demonstrated superior performance to conventional calcium phosphates and equivalence to autologous bone in pre-clinical bone healing models. This is related to their ability to form bone in soft tissues, without the addition of cells and growth factors. It is hypothesized that a specific innate immune response to submicron topography contributes to the enhanced bone healing by these materials. Upregulation of pro-healing, anti-inflammatory ‘M2’ macrophages versus pro-inflammatory ‘M1’ macrophages on submicron-structured calcium phosphates may be involved. In this in vitro study, the response of primary human macrophages to different calcium phosphate bone graft substitutes was assessed. Primary CD14+ monocytes were isolated from human buffy coats and were seeded on two different calcium phosphate materials. The first material had a submicron topography of needle-shaped crystals (BCP<μm) while the second material had no submicron topography (TCP). Macrophage M1/M2 phenotype characterization by protein and gene expression markers at 24 h and 72 h indicated overall stronger macrophage activation and subtle phenotypic skewing towards the M2 phenotype on BCP<μm vs TCP. Moreover, macrophages exhibited an elongated morphology on BCP<μm, which is associated with the M2 phenotype, while macrophages on TCP primarily exhibited a spherical morphology. Conditioned medium of macrophages cultured on BCP<μm resulted in enhanced in vitro angiogenic tube formation and osteogenic differentiation of mesenchymal stromal cells, compared to conditioned medium from macrophages on TCP. Altogether, these findings suggest a potential role of M2 macrophage upregulation in the bone-induction mechanism of calcium phosphates with submicron surface topography.