Lipid-lowering therapy is of utmost importance in both primary and secondary prevention of atherosclerotic cardio vascular disease (ASCVD). However, the presence of residual risk allows cardiovascular events to occur even when low-density lipoprotein cholesterol (LDL-C) levels are very low. A large number of clinical studies have provided evidence confirming the association between elevated plasma Lp(a) and the development of ASCVD. Clinical studies have also suggested that reducing Lp(a) may help decrease the occurrence of cardiovascular events.
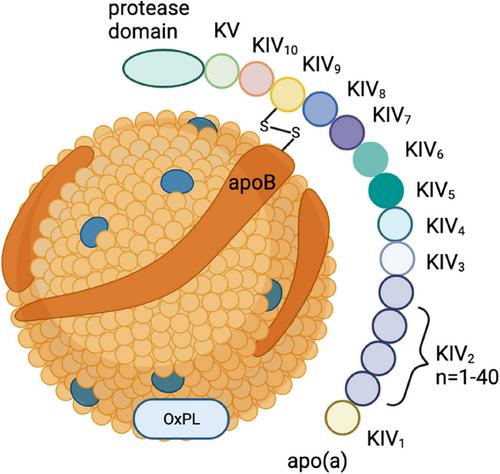
Lp(a) consists of LDL-like particles, apo(a) and OxPL. The level of Lp(a) in thehuman body is predominantly determined by genetics, with external factorshaving minimal impact. Additionally, Lp(a) levels have been found to vary among different ethnicities. There is a notable correlation between elevated levels of Lp(a) and coronary artery disease (CAD), which is independent of other lipoproteins. Furthermore, there exists a linear relationship between Lp(a) levels and the risk of developing ASCVD. It is now wildly believed that Lp(a) primarily contributes to the development of cardiovascular events through pro-inflammation, pro-thrombosis and pro-atherosclerosis. From the perspective of Lp(a) influencing inflammation, it primarily promotes the release of inflammatoryfactors. This, in turn, increases levels of vascular inflammation and facilitates the recruitment of monocytes-macrophages. Moreover, it also affects the function of endothelial cells during the development process of atherosclerosis. All these aspects complement each other and contribute to the progression of at herosclerosis. Currently, the lipid-lowering treatment used inclinical practice can partially reduce the levels of Lp(a), but its impact on inflammation is not significant.
Lp(a) is an independent risk factor for CAD, as it promotes inflammation in the body and accelerates theprogression of atherosclerosis. Further research on effective methods to reduce Lp(a) levels can provide new insights for the treatment of atherosclerosis.