Substantially Delayed Maturation of Growth Plate Chondrocytes in “Humanized” PTH1R Mice with the H223R Mutation of Jansen's Disease
Monica Reyes, Damla Firat, Patrick Hanna, Mohd Khan, Michael Bruce, Maria Shvedova, Tatsuya Kobayashi, Ernestina Schipani, Thomas J. Gardella, Harald Jüppner
下载PDF
{"title":"Substantially Delayed Maturation of Growth Plate Chondrocytes in “Humanized” PTH1R Mice with the H223R Mutation of Jansen's Disease","authors":"Monica Reyes, Damla Firat, Patrick Hanna, Mohd Khan, Michael Bruce, Maria Shvedova, Tatsuya Kobayashi, Ernestina Schipani, Thomas J. Gardella, Harald Jüppner","doi":"10.1002/jbm4.10802","DOIUrl":null,"url":null,"abstract":"<p>Activating parathyroid hormone (PTH)/PTH-related Peptide (PTHrP) receptor (PTH1R) mutations causes Jansen's metaphyseal chondrodysplasia (JMC), a rare disease characterized by growth plate abnormalities, short stature, and PTH-independent hypercalcemia. Previously generated transgenic JMC mouse models, in which the human <i>PTH1R</i> allele with the H223R mutation (<i>H223R-PTH1R</i>) is expressed in osteoblasts via type Ia1 collagen or DMP1 promoters cause excess bone mass, while expression of the mutant allele via the type IIa1 collagen promoter results in only minor growth plate changes. Thus, neither transgenic JMC model adequately recapitulates the human disease. We therefore generated “humanized” JMC mice in which the <i>H223R-PTH1R</i> allele was expressed via the endogenous mouse <i>Pth1r</i> promoter and, thus, in all relevant target tissues. Founders with the H223R allele typically died within 2 months without reproducing; several mosaic male founders, however, lived longer and produced F1 <i>H223R-PTH1R</i> offspring, which were small and exhibited marked growth plate abnormalities. Serum calcium and phosphate levels of the mutant mice were not different from wild-type littermates, but serum PTH and P1NP were reduced significantly, while CTX-1 and CTX-2 were slightly increased. Histological and RNAscope analyses of the mutant tibial growth plates revealed markedly expanded zones of type II collagen-positive, proliferating/prehypertrophic chondrocytes, abundant apoptotic cells in the growth plate center and a progressive reduction of type X collagen-positive hypertrophic chondrocytes and primary spongiosa. The “humanized” <i>H223R-PTH1R</i> mice are likely to provide a more suitable model for defining the JMC phenotype and for assessing potential treatment options for this debilitating disease of skeletal development and mineral ion homeostasis. © 2023 The Authors. <i>JBMR Plus</i> published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.</p>","PeriodicalId":14611,"journal":{"name":"JBMR Plus","volume":"7 10","pages":""},"PeriodicalIF":3.4000,"publicationDate":"2023-08-15","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/jbm4.10802","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"JBMR Plus","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/jbm4.10802","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"ENDOCRINOLOGY & METABOLISM","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
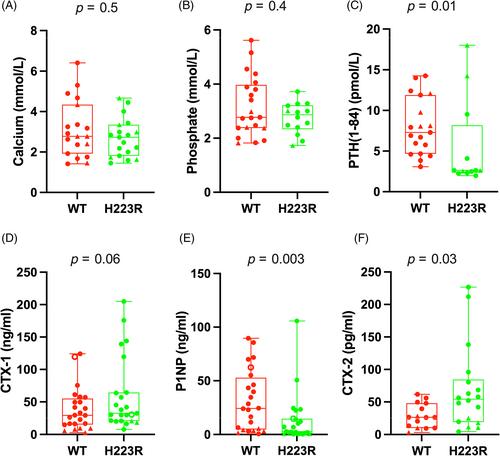
Activating parathyroid hormone (PTH)/PTH-related Peptide (PTHrP) receptor (PTH1R) mutations causes Jansen's metaphyseal chondrodysplasia (JMC), a rare disease characterized by growth plate abnormalities, short stature, and PTH-independent hypercalcemia. Previously generated transgenic JMC mouse models, in which the human PTH1R allele with the H223R mutation (H223R-PTH1R ) is expressed in osteoblasts via type Ia1 collagen or DMP1 promoters cause excess bone mass, while expression of the mutant allele via the type IIa1 collagen promoter results in only minor growth plate changes. Thus, neither transgenic JMC model adequately recapitulates the human disease. We therefore generated “humanized” JMC mice in which the H223R-PTH1R allele was expressed via the endogenous mouse Pth1r promoter and, thus, in all relevant target tissues. Founders with the H223R allele typically died within 2 months without reproducing; several mosaic male founders, however, lived longer and produced F1 H223R-PTH1R offspring, which were small and exhibited marked growth plate abnormalities. Serum calcium and phosphate levels of the mutant mice were not different from wild-type littermates, but serum PTH and P1NP were reduced significantly, while CTX-1 and CTX-2 were slightly increased. Histological and RNAscope analyses of the mutant tibial growth plates revealed markedly expanded zones of type II collagen-positive, proliferating/prehypertrophic chondrocytes, abundant apoptotic cells in the growth plate center and a progressive reduction of type X collagen-positive hypertrophic chondrocytes and primary spongiosa. The “humanized” H223R-PTH1R mice are likely to provide a more suitable model for defining the JMC phenotype and for assessing potential treatment options for this debilitating disease of skeletal development and mineral ion homeostasis. © 2023 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Jansen病H223R突变的“人源化”PTH1R小鼠生长板软骨细胞的成熟显著延迟
激活甲状旁腺激素(PTH)/PTH相关肽(PTHrP)受体(PTH1R)突变会导致詹森干骺端软骨发育不良(JMC),这是一种罕见的疾病,以生长板异常、身材矮小和PTH非依赖性高钙血症为特征。先前产生的转基因JMC小鼠模型,其中具有H223R突变的人PTH1R等位基因(H223R-PTH1R)通过Ia1型胶原或DMP1启动子在成骨细胞中表达,导致骨量过多,而突变等位基因通过IIa1型胶原启动子的表达仅导致生长板的微小变化。因此,两个转基因JMC模型都不能充分概括人类疾病。因此,我们产生了“人源化”JMC小鼠,其中H223R-PTH1R等位基因通过内源性小鼠PTH1R启动子表达,从而在所有相关靶组织中表达。具有H223R等位基因的创始人通常在2 数月未繁殖;然而,几个马赛克雄性创始人寿命更长,产生了F1 H223R-PTH1R后代,这些后代很小,并表现出明显的生长板异常。突变小鼠的血清钙和磷酸盐水平与野生型同窝出生的小鼠没有差异,但血清PTH和P1NP显著降低,而CTX-1和CTX-2略有增加。突变胫骨生长板的组织学和RNAscope分析显示,II型胶原阳性、增殖/肥大前软骨细胞的区域明显扩大,生长板中心有大量凋亡细胞,X型胶原阳性肥大软骨细胞和原发性海绵状软骨细胞逐渐减少。“人源化”H223R-PTH1R小鼠可能提供一个更合适的模型来定义JMC表型,并评估这种骨骼发育和矿物离子稳态的衰弱性疾病的潜在治疗选择。©2023作者。由Wiley Periodicals LLC代表美国骨与矿物研究学会出版的JBMR Plus。
本文章由计算机程序翻译,如有差异,请以英文原文为准。