Metabolic shift underlies tumor progression and immune evasion in S-nitrosoglutathione reductase-deficient cancer
Rafael Mena-Osuna, Ana Mantrana, Silvia Guil-Luna, María Teresa Sánchez-Montero, Carmen Navarrete-Sirvent, Teresa Morales-Ruiz, Aurora Rivas-Crespo, Marta Toledano-Fonseca, María Victoria García-Ortíz, Gema García-Jurado, María Auxiliadora Gómez-España, Rafael González-Fernández, Carlos Villar, Francisco Javier Medina-Fernández, José Manuel Villalba, Enrique Aranda, Antonio Rodríguez-Ariza
下载PDF
{"title":"Metabolic shift underlies tumor progression and immune evasion in S-nitrosoglutathione reductase-deficient cancer","authors":"Rafael Mena-Osuna, Ana Mantrana, Silvia Guil-Luna, María Teresa Sánchez-Montero, Carmen Navarrete-Sirvent, Teresa Morales-Ruiz, Aurora Rivas-Crespo, Marta Toledano-Fonseca, María Victoria García-Ortíz, Gema García-Jurado, María Auxiliadora Gómez-España, Rafael González-Fernández, Carlos Villar, Francisco Javier Medina-Fernández, José Manuel Villalba, Enrique Aranda, Antonio Rodríguez-Ariza","doi":"10.1002/path.6080","DOIUrl":null,"url":null,"abstract":"<p>S-nitrosoglutathione reductase (GSNOR) is a denitrosylase enzyme that has been suggested to play a tumor suppressor role, although the mechanisms responsible are still largely unclear. In this study, we show that GSNOR deficiency in tumors is associated with poor prognostic histopathological features and poor survival in patients with colorectal cancer (CRC). GSNOR-low tumors were characterized by an immunosuppressive microenvironment with exclusion of cytotoxic CD8<sup>+</sup> T cells. Notably, GSNOR-low tumors exhibited an immune evasive proteomic signature along with an altered energy metabolism characterized by impaired oxidative phosphorylation (OXPHOS) and energetic dependence on glycolytic activity. CRISPR-Cas9-mediated generation of GSNOR gene knockout (KO) CRC cells confirmed <i>in vitro</i> and <i>in vivo</i> that GSNOR-deficiency conferred higher tumorigenic and tumor-initiating capacities. Moreover, GSNOR-KO cells possessed enhanced immune evasive properties and resistance to immunotherapy, as revealed following xenografting them into humanized mouse models. Importantly, GSNOR-KO cells were characterized by a metabolic shift from OXPHOS to glycolysis to produce energy, as indicated by increased lactate secretion, higher sensitivity to 2-deoxyglucose (2DG), and a fragmented mitochondrial network. Real-time metabolic analysis revealed that GSNOR-KO cells operated close to their maximal glycolytic rate, as a compensation for lower OXPHOS levels, explaining their higher sensitivity to 2DG. Remarkably, this higher susceptibility to glycolysis inhibition with 2DG was validated in patient-derived xenografts and organoids from clinical GSNOR-low tumors. In conclusion, our data support the idea that metabolic reprogramming induced by GSNOR deficiency is an important mechanism for tumor progression and immune evasion in CRC and that the metabolic vulnerabilities associated with the deficiency of this denitrosylase can be exploited therapeutically. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"260 3","pages":"261-275"},"PeriodicalIF":5.2000,"publicationDate":"2023-04-05","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6080","citationCount":"1","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6080","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 1
引用
批量引用
Abstract
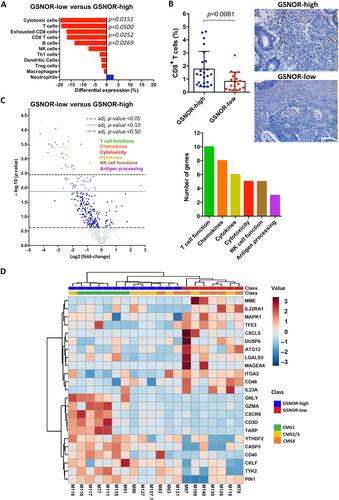
S-nitrosoglutathione reductase (GSNOR) is a denitrosylase enzyme that has been suggested to play a tumor suppressor role, although the mechanisms responsible are still largely unclear. In this study, we show that GSNOR deficiency in tumors is associated with poor prognostic histopathological features and poor survival in patients with colorectal cancer (CRC). GSNOR-low tumors were characterized by an immunosuppressive microenvironment with exclusion of cytotoxic CD8+ T cells. Notably, GSNOR-low tumors exhibited an immune evasive proteomic signature along with an altered energy metabolism characterized by impaired oxidative phosphorylation (OXPHOS) and energetic dependence on glycolytic activity. CRISPR-Cas9-mediated generation of GSNOR gene knockout (KO) CRC cells confirmed in vitro and in vivo that GSNOR-deficiency conferred higher tumorigenic and tumor-initiating capacities. Moreover, GSNOR-KO cells possessed enhanced immune evasive properties and resistance to immunotherapy, as revealed following xenografting them into humanized mouse models. Importantly, GSNOR-KO cells were characterized by a metabolic shift from OXPHOS to glycolysis to produce energy, as indicated by increased lactate secretion, higher sensitivity to 2-deoxyglucose (2DG), and a fragmented mitochondrial network. Real-time metabolic analysis revealed that GSNOR-KO cells operated close to their maximal glycolytic rate, as a compensation for lower OXPHOS levels, explaining their higher sensitivity to 2DG. Remarkably, this higher susceptibility to glycolysis inhibition with 2DG was validated in patient-derived xenografts and organoids from clinical GSNOR-low tumors. In conclusion, our data support the idea that metabolic reprogramming induced by GSNOR deficiency is an important mechanism for tumor progression and immune evasion in CRC and that the metabolic vulnerabilities associated with the deficiency of this denitrosylase can be exploited therapeutically. © 2023 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
代谢转移是s -亚硝基谷胱甘肽还原酶缺乏癌症的肿瘤进展和免疫逃避的基础
S-亚硝基谷胱甘肽还原酶(GSNOR)是一种反糖基酶,已被认为发挥肿瘤抑制作用,尽管其机制仍不清楚。在这项研究中,我们发现肿瘤中GSNOR缺乏与癌症(CRC)患者预后不良的组织病理学特征和生存率低有关。GSNOR低肿瘤的特征是免疫抑制微环境排除了细胞毒性CD8+T细胞。值得注意的是,GSNOR低肿瘤表现出免疫逃避蛋白质组学特征,以及以氧化磷酸化受损(OXPHOS)和对糖酵解活性的能量依赖为特征的能量代谢改变。CRISPR-Cas9介导的GSNOR基因敲除(KO)CRC细胞的产生在体外和体内证实,GSNOR缺乏具有更高的致瘤和肿瘤启动能力。此外,GSNOR-KO细胞具有增强的免疫逃避特性和对免疫疗法的抵抗力,这在将其异种移植到人源化小鼠模型中后得到了揭示。重要的是,GSNOR-KO细胞的特征是从OXPHOS到糖酵解的代谢转变以产生能量,如乳酸分泌增加、对2-脱氧葡萄糖(2DG)的敏感性更高和线粒体网络碎片化所示。实时代谢分析显示,GSNOR-KO细胞在接近其最大糖酵解速率的情况下运行,作为对较低OXPHOS水平的补偿,解释了它们对2DG的较高敏感性。值得注意的是,2DG对糖酵解抑制的更高易感性在来自临床GSNOR低肿瘤的患者来源的异种移植物和类器官中得到了验证。总之,我们的数据支持这样一种观点,即GSNOR缺乏诱导的代谢重编程是CRC中肿瘤进展和免疫逃避的重要机制,并且与这种反糖基酶缺乏相关的代谢脆弱性可以用于治疗。©2023作者。病理学杂志由John Wiley&;代表大不列颠及爱尔兰病理学会的Sons有限公司。
本文章由计算机程序翻译,如有差异,请以英文原文为准。