Anti-CD40L therapy prevents the formation of precursor lesions to gastric B-cell MALT lymphoma in a mouse model
Le Ying, Phoebe Liu, Zhoujie Ding, Georgie Wray-McCann, Jack Emery, Nina Colon, Lena HM Le, Le Son Tran, Ping Xu, Liang Yu, Dana J Philpott, Yugang Tu, Daryl MZ Cheah, Chee L Cheng, Soon T Lim, Choon K Ong, Richard L Ferrero
下载PDF
{"title":"Anti-CD40L therapy prevents the formation of precursor lesions to gastric B-cell MALT lymphoma in a mouse model","authors":"Le Ying, Phoebe Liu, Zhoujie Ding, Georgie Wray-McCann, Jack Emery, Nina Colon, Lena HM Le, Le Son Tran, Ping Xu, Liang Yu, Dana J Philpott, Yugang Tu, Daryl MZ Cheah, Chee L Cheng, Soon T Lim, Choon K Ong, Richard L Ferrero","doi":"10.1002/path.6053","DOIUrl":null,"url":null,"abstract":"<p>Mucosa-associated lymphoid tissue (MALT) lymphoma is a B-cell tumour that develops over many decades in the stomachs of individuals with chronic <i>Helicobacter pylori</i> infection. We developed a new mouse model of human gastric MALT lymphoma in which mice with a myeloid-specific deletion of the innate immune molecule, Nlrc5, develop precursor B-cell lesions to MALT lymphoma at only 3 months post-<i>Helicobacter</i> infection versus 9–24 months in existing models. The gastric B-cell lesions in the <i>Nlrc5</i> knockout mice had the histopathological features of the human disease, notably lymphoepithelial-like lesions, centrocyte-like cells, and were infiltrated by dendritic cells (DCs), macrophages, and T-cells (CD4<sup>+</sup>, CD8<sup>+</sup> and Foxp3<sup>+</sup>). Mouse and human gastric tissues contained immune cells expressing immune checkpoint receptor programmed death 1 (PD-1) and its ligand PD-L1, indicating an immunosuppressive tissue microenvironment. We next determined whether CD40L, overexpressed in a range of B-cell malignancies, may be a potential drug target for the treatment of gastric MALT lymphoma. Importantly, we showed that the administration of anti-CD40L antibody either coincident with or after establishment of <i>Helicobacter</i> infection prevented gastric B-cell lesions in mice, when compared with the control antibody treatment. Mice administered the CD40L antibody also had significantly reduced numbers of gastric DCs, CD8<sup>+</sup> and Foxp3<sup>+</sup> T-cells, as well as decreased gastric expression of B-cell lymphoma genes. These findings validate the potential of CD40L as a therapeutic target in the treatment of human gastric B-cell MALT lymphoma. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"259 4","pages":"402-414"},"PeriodicalIF":5.2000,"publicationDate":"2023-01-14","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6053","citationCount":"1","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6053","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 1
引用
批量引用
Abstract
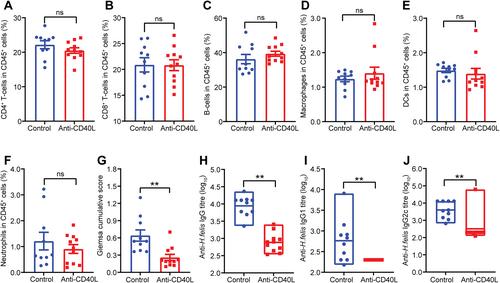
Mucosa-associated lymphoid tissue (MALT) lymphoma is a B-cell tumour that develops over many decades in the stomachs of individuals with chronic Helicobacter pylori infection. We developed a new mouse model of human gastric MALT lymphoma in which mice with a myeloid-specific deletion of the innate immune molecule, Nlrc5, develop precursor B-cell lesions to MALT lymphoma at only 3 months post-Helicobacter infection versus 9–24 months in existing models. The gastric B-cell lesions in the Nlrc5 knockout mice had the histopathological features of the human disease, notably lymphoepithelial-like lesions, centrocyte-like cells, and were infiltrated by dendritic cells (DCs), macrophages, and T-cells (CD4+ , CD8+ and Foxp3+ ). Mouse and human gastric tissues contained immune cells expressing immune checkpoint receptor programmed death 1 (PD-1) and its ligand PD-L1, indicating an immunosuppressive tissue microenvironment. We next determined whether CD40L, overexpressed in a range of B-cell malignancies, may be a potential drug target for the treatment of gastric MALT lymphoma. Importantly, we showed that the administration of anti-CD40L antibody either coincident with or after establishment of Helicobacter infection prevented gastric B-cell lesions in mice, when compared with the control antibody treatment. Mice administered the CD40L antibody also had significantly reduced numbers of gastric DCs, CD8+ and Foxp3+ T-cells, as well as decreased gastric expression of B-cell lymphoma genes. These findings validate the potential of CD40L as a therapeutic target in the treatment of human gastric B-cell MALT lymphoma. © 2023 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.