下载PDF
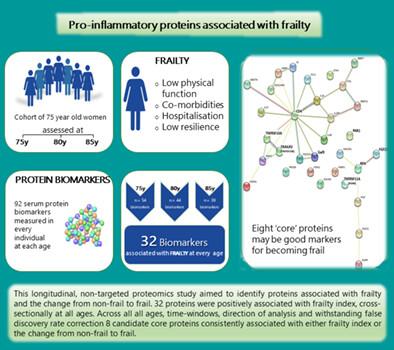
{"title":"Pro-Inflammatory Proteins Associated with Frailty and Its Progression—A Longitudinal Study in Community-Dwelling Women","authors":"Adam Mitchell, Linnea Malmgren, Patrik Bartosch, Fiona Elizabeth McGuigan, Kristina E. Akesson","doi":"10.1002/jbmr.4861","DOIUrl":null,"url":null,"abstract":"<p>The complex pathophysiology underlying biological aging creates challenges for identifying biomarkers associated with frailty. This longitudinal, nontargeted proteomics study aimed to identify proteins associated with frailty, particularly the change from nonfrail to frail. The population-based Osteoporosis Prospective Risk Assessment cohort includes women all of whom are 75 years old at inclusion (<i>n</i> = 1044) and reassessed at 80 years (<i>n</i> = 715) and 85 years (<i>n</i> = 382). A deficits in health frailty index (FI) and 92 plasma proteins (Olink CVD-II panel) were available at all ages. The identical age facilitated differentiating chronological and biological aging. Bidirectional analyses, performed cross-sectionally and longitudinally, used regression models controlled for false discovery rate (FDR), across 5- and 10-year time windows and longitudinal mixed models. Frailty outcomes were frailty index, frailty status (frail defined as FI ≥ 0.25), change in frailty index, and change in frailty status, together with protein expression or change in protein expression. Elevated levels of 32 proteins were positively associated with the FI, cross-sectionally at all ages (range: β-coefficients 0.22–2.06; FDR 0.021–0.024), of which 18 were also associated with frailty status (range: odds ratios 1.40–5.77; FDR 0.022–0.016). Based on the accrued data, eight core proteins (CD4, FGF23, Gal-9, PAR-1, REN, TNFRSF10A TNFRSF11A, and TNFRSF10B) are proposed. A one-unit change in the FI was additively associated with increased protein expression over 5 and 10 years (range: β-coefficients 0.52–1.59; <i>p</i> < 0.001). Increments in baseline FI consistently associated with a change in protein expression over time (5 years, β-range 0.05–1.35; 10 years, β-range 0.51–1.48; all <i>p</i> < 0.001). A one-unit increase in protein expression was also associated with an increased probability of being frail (FI ≥ 0.25) (β-range: 0.14–0.61). Mirroring the multisystem deterioration that typifies frailty, the proteins and their associated biological pathways reflect pathologies, including the renal system, skeletal homeostasis, and TRAIL-activated apoptotic signaling. The core proteins are compelling candidates for understanding the development and progression of frailty with advancing age, including the intrinsic musculoskeletal component. © 2023 The Authors. <i>Journal of Bone and Mineral Research</i> published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).</p>","PeriodicalId":185,"journal":{"name":"Journal of Bone and Mineral Research","volume":"38 8","pages":"1076-1091"},"PeriodicalIF":5.9000,"publicationDate":"2023-05-30","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/jbmr.4861","citationCount":"1","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Journal of Bone and Mineral Research","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/jbmr.4861","RegionNum":1,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ENDOCRINOLOGY & METABOLISM","Score":null,"Total":0}
引用次数: 1
引用
批量引用
Abstract
The complex pathophysiology underlying biological aging creates challenges for identifying biomarkers associated with frailty. This longitudinal, nontargeted proteomics study aimed to identify proteins associated with frailty, particularly the change from nonfrail to frail. The population-based Osteoporosis Prospective Risk Assessment cohort includes women all of whom are 75 years old at inclusion (n = 1044) and reassessed at 80 years (n = 715) and 85 years (n = 382). A deficits in health frailty index (FI) and 92 plasma proteins (Olink CVD-II panel) were available at all ages. The identical age facilitated differentiating chronological and biological aging. Bidirectional analyses, performed cross-sectionally and longitudinally, used regression models controlled for false discovery rate (FDR), across 5- and 10-year time windows and longitudinal mixed models. Frailty outcomes were frailty index, frailty status (frail defined as FI ≥ 0.25), change in frailty index, and change in frailty status, together with protein expression or change in protein expression. Elevated levels of 32 proteins were positively associated with the FI, cross-sectionally at all ages (range: β-coefficients 0.22–2.06; FDR 0.021–0.024), of which 18 were also associated with frailty status (range: odds ratios 1.40–5.77; FDR 0.022–0.016). Based on the accrued data, eight core proteins (CD4, FGF23, Gal-9, PAR-1, REN, TNFRSF10A TNFRSF11A, and TNFRSF10B) are proposed. A one-unit change in the FI was additively associated with increased protein expression over 5 and 10 years (range: β-coefficients 0.52–1.59; p < 0.001). Increments in baseline FI consistently associated with a change in protein expression over time (5 years, β-range 0.05–1.35; 10 years, β-range 0.51–1.48; all p < 0.001). A one-unit increase in protein expression was also associated with an increased probability of being frail (FI ≥ 0.25) (β-range: 0.14–0.61). Mirroring the multisystem deterioration that typifies frailty, the proteins and their associated biological pathways reflect pathologies, including the renal system, skeletal homeostasis, and TRAIL-activated apoptotic signaling. The core proteins are compelling candidates for understanding the development and progression of frailty with advancing age, including the intrinsic musculoskeletal component. © 2023 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).