Intestinal mucositis is the major side effect during abdominal or pelvic radiotherapy, but the underlying immunogen remains to be further characterised and few radioprotective agents are available. This study investigated the role of dsDNA-triggered inflammasomes in intestinal mucositis during radiotherapy.
Pro-inflammatory cytokines were detected by ELISA. Radiation-induced intestinal injury in mice was analyzed by means of survival curves, body weight, HE staining of intestines, and intestinal barrier integrity. Western blot, immunofluorescence staining, co-immunoprecipitation assay and flow cytometry were used to investigate the regulatory role of dsDNA on inflammasomes.
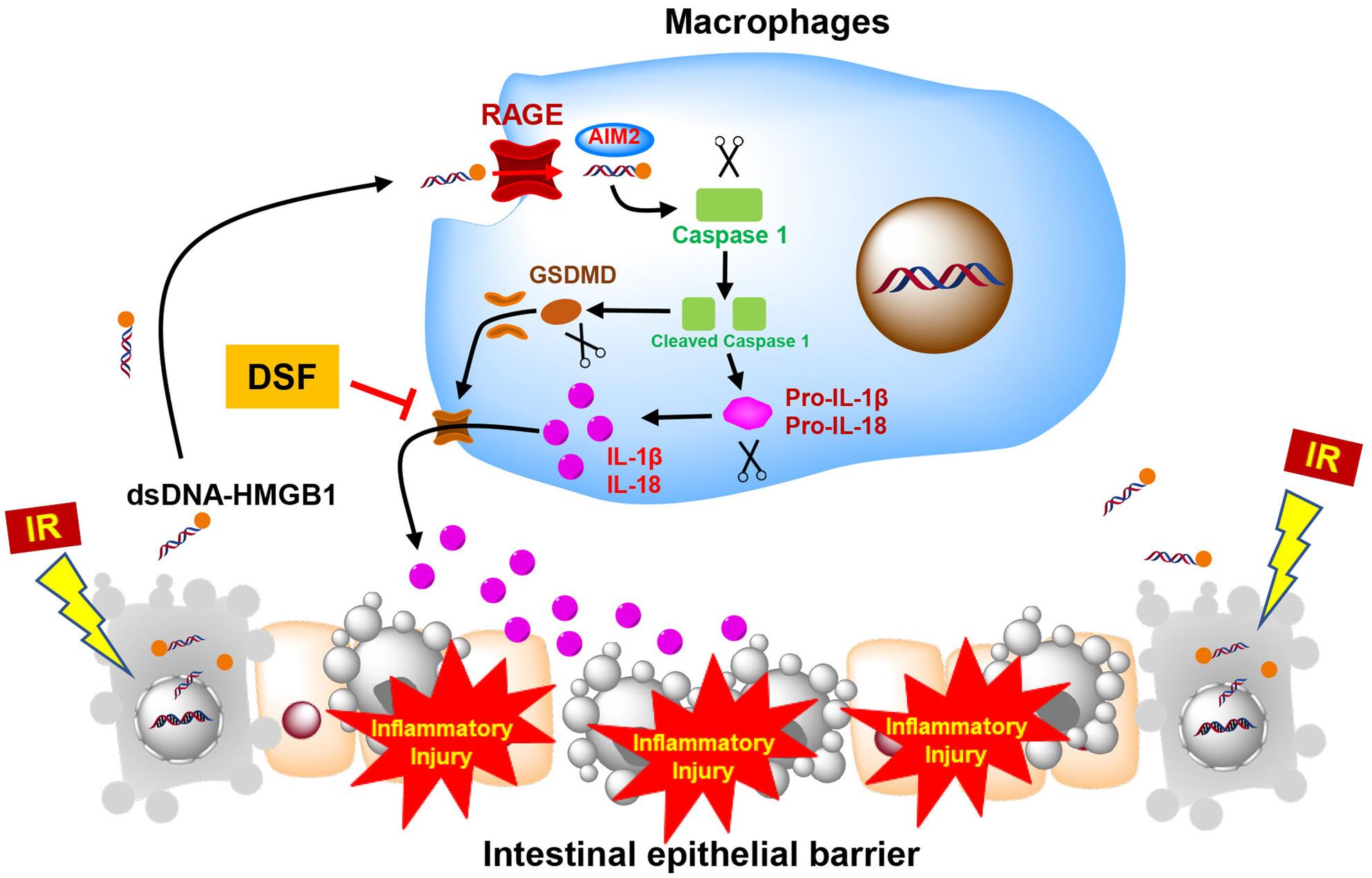
Here, we show that a high level of IL-1β and IL-18 is associated with diarrhoea in colorectal cancer (CRC) patients during radiotherapy, which accounts for intestinal radiotoxicity. Subsequently, we found that the dose-dependently released dsDNA from the intestinal epithelial cells (IECs) serves as the potential immunogenic molecule for radiation-induced intestinal mucositis. Our results further indicate that the released dsDNA transfers into the macrophages in an HMGB1/RAGE-dependent manner and then triggers absent in melanoma 2 (AIM2) inflammasome activation and the IL-1β and IL-18 secretion. Finally, we show that the FDA-approved disulfiram (DSF), a newly identified inflammasome inhibitor, could mitigate intestinal radiotoxicity by controlling inflammasome.
These findings indicate that the extracellular self-dsDNA released from the irradiated IECs is a potential immunogen to stimulate immune cells and trigger the subsequent intestinal mucositis, while blunting the dsDNA-triggered inflammasome in macrophages may represent an exciting therapeutic strategy for side effects control during abdominal radiotherapy.