Intravascular hemolysis triggers NAFLD characterized by a deregulation of lipid metabolism and lipophagy blockade
Sandra Rayego-Mateos, José Luis Morgado-Pascual, Cristina García-Caballero, Iolanda Lazaro, Aleix Sala-Vila, Lucas Opazo-Rios, Sebastian Mas-Fontao, Jesús Egido, Marta Ruiz-Ortega, Juan Antonio Moreno
下载PDF
{"title":"Intravascular hemolysis triggers NAFLD characterized by a deregulation of lipid metabolism and lipophagy blockade","authors":"Sandra Rayego-Mateos, José Luis Morgado-Pascual, Cristina García-Caballero, Iolanda Lazaro, Aleix Sala-Vila, Lucas Opazo-Rios, Sebastian Mas-Fontao, Jesús Egido, Marta Ruiz-Ortega, Juan Antonio Moreno","doi":"10.1002/path.6161","DOIUrl":null,"url":null,"abstract":"<p>Intravascular hemolysis is a common feature of different clinical entities, including sickle cell disease and malaria. Chronic hemolytic disorders are associated with hepatic damage; however, it is unknown whether heme disturbs lipid metabolism and promotes liver steatosis, thereby favoring the progression to nonalcoholic fatty liver disease (NAFLD). Using an experimental model of acute intravascular hemolysis, we report here the presence of liver injury in association with microvesicular lipid droplet deposition. Hemolysis promoted serum hyperlipidemia and altered intrahepatic triglyceride fatty acid composition, with increments in oleic, palmitoleic, and palmitic acids. These findings were related to augmented expression of transporters involved in fatty acid uptake (CD36 and MSR1) and deregulation of LDL transport, as demonstrated by decreased levels of LDL receptor and increased PCSK9 expression. Hemolysis also upregulated hepatic enzymes associated with cholesterol biosynthesis (SREBP2, HMGC1, LCAT, SOAT1) and transcription factors regulating lipid metabolism (SREBP1). Increased LC3II/LC3I ratio and p62/SQSTM1 protein levels were reported in mice with intravascular hemolysis and hepatocytes stimulated with heme, indicating a blockade of lipophagy. In cultured hepatocytes, cell pretreatment with the autophagy inductor rapamycin diminished heme-mediated toxicity and accumulation of lipid droplets. In conclusion, intravascular hemolysis enhances liver damage by exacerbating lipid accumulation and blocking the lipophagy pathway, thereby promoting NAFLD. These new findings have a high translational potential as a novel NAFLD-promoting mechanism in individuals suffering from severe hemolysis episodes. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"261 2","pages":"169-183"},"PeriodicalIF":5.2000,"publicationDate":"2023-08-09","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6161","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6161","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
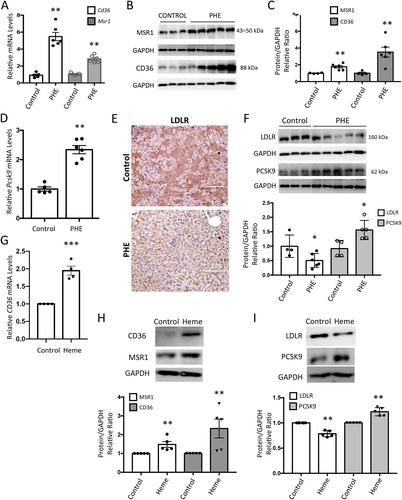
Intravascular hemolysis is a common feature of different clinical entities, including sickle cell disease and malaria. Chronic hemolytic disorders are associated with hepatic damage; however, it is unknown whether heme disturbs lipid metabolism and promotes liver steatosis, thereby favoring the progression to nonalcoholic fatty liver disease (NAFLD). Using an experimental model of acute intravascular hemolysis, we report here the presence of liver injury in association with microvesicular lipid droplet deposition. Hemolysis promoted serum hyperlipidemia and altered intrahepatic triglyceride fatty acid composition, with increments in oleic, palmitoleic, and palmitic acids. These findings were related to augmented expression of transporters involved in fatty acid uptake (CD36 and MSR1) and deregulation of LDL transport, as demonstrated by decreased levels of LDL receptor and increased PCSK9 expression. Hemolysis also upregulated hepatic enzymes associated with cholesterol biosynthesis (SREBP2, HMGC1, LCAT, SOAT1) and transcription factors regulating lipid metabolism (SREBP1). Increased LC3II/LC3I ratio and p62/SQSTM1 protein levels were reported in mice with intravascular hemolysis and hepatocytes stimulated with heme, indicating a blockade of lipophagy. In cultured hepatocytes, cell pretreatment with the autophagy inductor rapamycin diminished heme-mediated toxicity and accumulation of lipid droplets. In conclusion, intravascular hemolysis enhances liver damage by exacerbating lipid accumulation and blocking the lipophagy pathway, thereby promoting NAFLD. These new findings have a high translational potential as a novel NAFLD-promoting mechanism in individuals suffering from severe hemolysis episodes. © 2023 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
血管内溶血触发NAFLD的特点是脂质代谢的解除和脂质吞噬阻断
血管内溶血是不同临床实体的共同特征,包括镰状细胞病和疟疾。慢性溶血性疾病与肝脏损伤有关;然而,尚不清楚血红素是否干扰脂质代谢并促进肝脏脂肪变性,从而有利于发展为非酒精性脂肪性肝病(NAFLD)。使用急性血管内溶血的实验模型,我们在这里报告了与微泡脂滴沉积相关的肝损伤的存在。溶血促进血清高脂血症,并改变肝内甘油三酯脂肪酸组成,油酸、棕榈油酸和棕榈酸增加。这些发现与参与脂肪酸摄取的转运蛋白(CD36和MSR1)的表达增加和LDL转运的失调有关,如LDL受体水平降低和PCSK9表达增加所示。溶血还上调了与胆固醇生物合成相关的肝酶(SREBP2、HMGC1、LCAT、SOAT1)和调节脂质代谢的转录因子(SREBP1)。据报道,在血管内溶血的小鼠和用血红素刺激的肝细胞中,LC3II/LC3I比率和p62/SQSTM1蛋白水平增加,表明脂吞噬被阻断。在培养的肝细胞中,用自噬诱导剂雷帕霉素预处理细胞可以减少血红素介导的毒性和脂滴的积累。总之,血管内溶血通过加剧脂质积聚和阻断脂吞噬途径来增强肝脏损伤,从而促进NAFLD。这些新发现作为一种新的NAFLD促进机制,在患有严重溶血发作的个体中具有很高的转化潜力。©2023作者。病理学杂志由John Wiley&;代表大不列颠及爱尔兰病理学会的Sons有限公司。
本文章由计算机程序翻译,如有差异,请以英文原文为准。