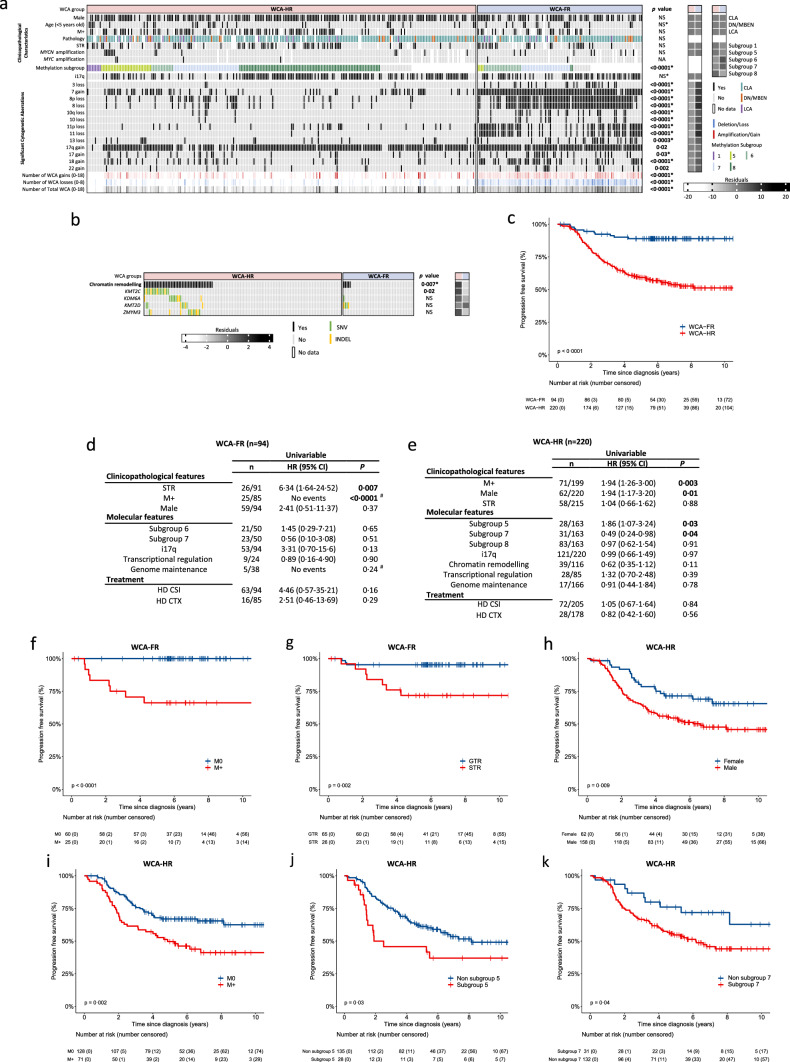
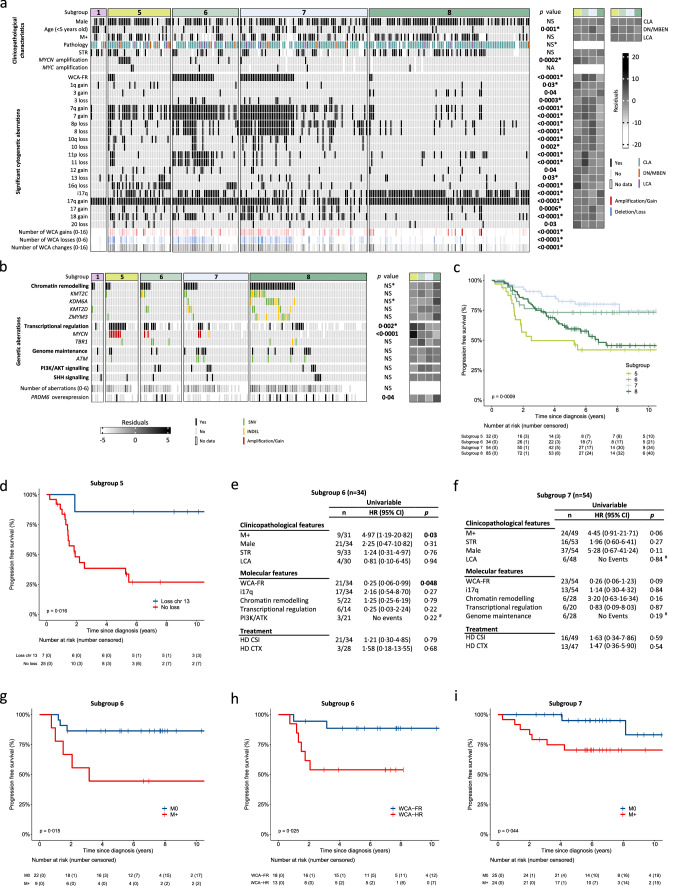
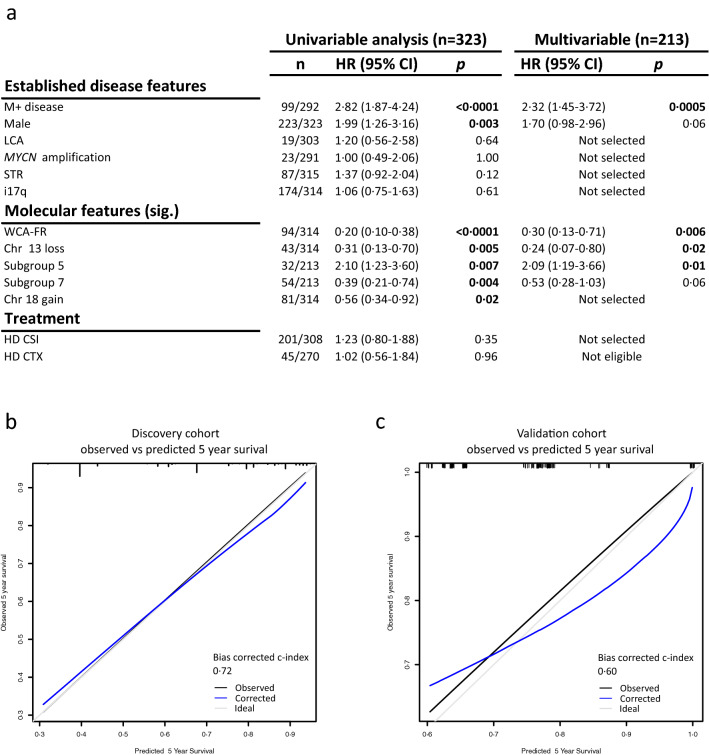
Group 4 tumours (MBGrp4) represent the majority of non-WNT/non-SHH medulloblastomas. Their clinical course is poorly predicted by current risk-factors. MBGrp4 molecular substructures have been identified (e.g. subgroups/cytogenetics/mutations), however their inter-relationships and potential to improve clinical sub-classification and risk-stratification remain undefined. We comprehensively characterised the paediatric MBGrp4 molecular landscape and determined its utility to improve clinical management. A clinically-annotated discovery cohort (n = 362 MBGrp4) was assembled from UK-CCLG institutions and SIOP-UKCCSG-PNET3, HIT-SIOP-PNET4 and PNET HR + 5 clinical trials. Molecular profiling was undertaken, integrating driver mutations, second-generation non-WNT/non-SHH subgroups (1–8) and whole-chromosome aberrations (WCAs). Survival models were derived for patients ≥ 3 years of age who received contemporary multi-modal therapies (n = 323). We first independently derived and validated a favourable-risk WCA group (WCA-FR) characterised by ≥ 2 features from chromosome 7 gain, 8 loss, and 11 loss. Remaining patients were high-risk (WCA-HR). Subgroups 6 and 7 were enriched for WCA-FR (p < 0·0001) and aneuploidy. Subgroup 8 was defined by predominantly balanced genomes with isolated isochromosome 17q (p < 0·0001). While no mutations were associated with outcome and overall mutational burden was low, WCA-HR harboured recurrent chromatin remodelling mutations (p = 0·007). Integration of methylation and WCA groups improved risk-stratification models and outperformed established prognostication schemes. Our MBGrp4 risk-stratification scheme defines: favourable-risk (non-metastatic disease and (i) subgroup 7 or (ii) WCA-FR (21% of patients, 5-year PFS 97%)), very-high-risk (metastatic disease with WCA-HR (36%, 5-year PFS 49%)) and high-risk (remaining patients; 43%, 5-year PFS 67%). These findings validated in an independent MBGrp4 cohort (n = 668). Importantly, our findings demonstrate that previously established disease-wide risk-features (i.e. LCA histology and MYC(N) amplification) have little prognostic relevance in MBGrp4 disease. Novel validated survival models, integrating clinical features, methylation and WCA groups, improve outcome prediction and re-define risk-status for ~ 80% of MBGrp4. Our MBGrp4 favourable-risk group has MBWNT-like excellent outcomes, thereby doubling the proportion of medulloblastoma patients who could benefit from therapy de-escalation approaches, aimed at reducing treatment induced late-effects while sustaining survival outcomes. Novel approaches are urgently required for the very-high-risk patients.