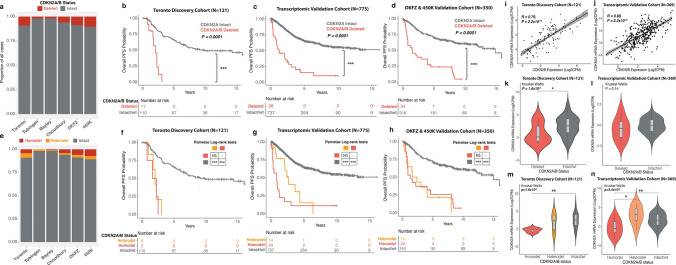
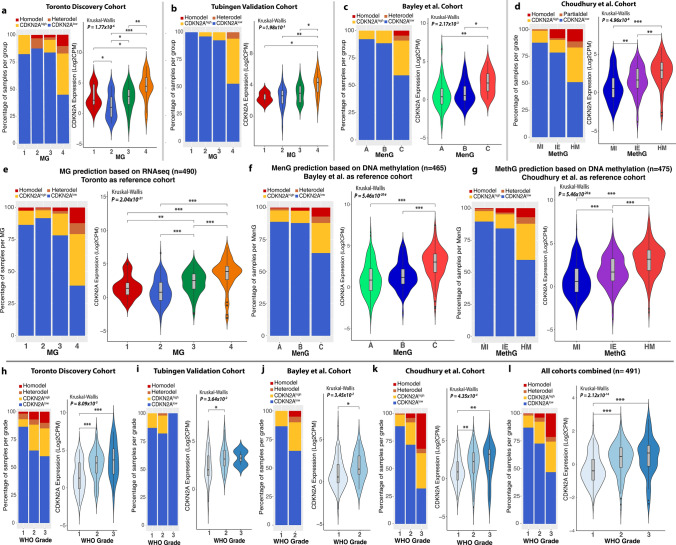
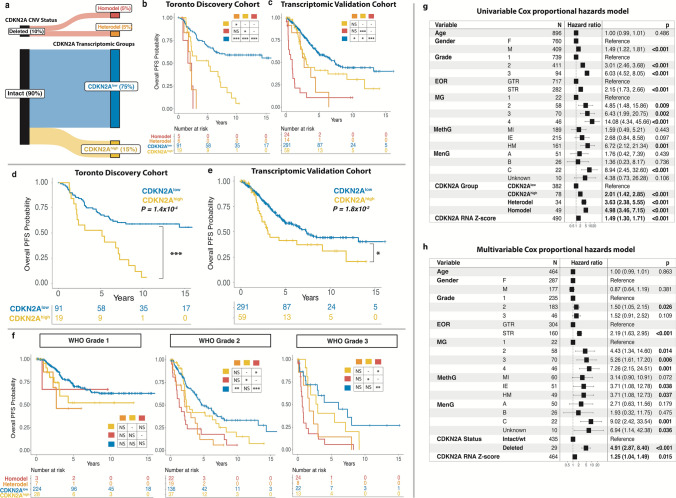
Homozygous deletion of CDKN2A/B was recently incorporated into the World Health Organization classification for grade 3 meningiomas. While this marker is overall rare in meningiomas, its relationship to other CDKN2A alterations on a transcriptomic, epigenomic, and copy number level has not yet been determined. We therefore utilized multidimensional molecular data of 1577 meningioma samples from 6 independent cohorts enriched for clinically aggressive meningiomas to comprehensively interrogate the spectrum of CDKN2A alterations through DNA methylation, copy number variation, transcriptomics, and proteomics using an integrated molecular approach. Homozygous CDKN2A/B deletions were identified in only 7.1% of cases but were associated with significantly poorer outcomes compared to tumors without these deletions. Heterozygous CDKN2A/B deletions were identified in 2.6% of cases and had similarly poor outcomes as those with homozygous deletions. Among tumors with intact CDKN2A/B (without a homozygous or heterozygous deletion), we found a distinct difference in outcome based on mRNA expression of CDKN2A, with meningiomas that had elevated mRNA expression (CDKN2Ahigh) having a significantly shorter time to recurrence. The expression of CDKN2A was independently prognostic after accounting for copy number loss and consistently increased with WHO grade and more aggressive molecular and methylation groups irrespective of cohort. Despite the discordant and mutually exclusive status of the CDKN2A gene in these groups, both CDKN2Ahigh meningiomas and meningiomas with CDKN2A deletions were enriched for similar cell cycle pathways but at different checkpoints. High mRNA expression of CDKN2A was also associated with gene hypermethylation, Rb-deficiency, and lack of response to CDK inhibition. p16 immunohistochemistry could not reliably differentiate between meningiomas with and without CDKN2A deletions but appeared to correlate better with mRNA expression. These findings support the role of CDKN2A mRNA expression as a biomarker of clinically aggressive meningiomas with potential therapeutic implications.