下载PDF
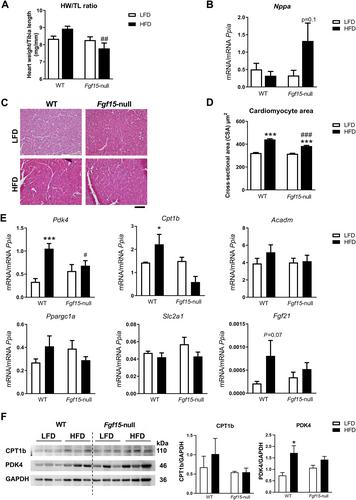
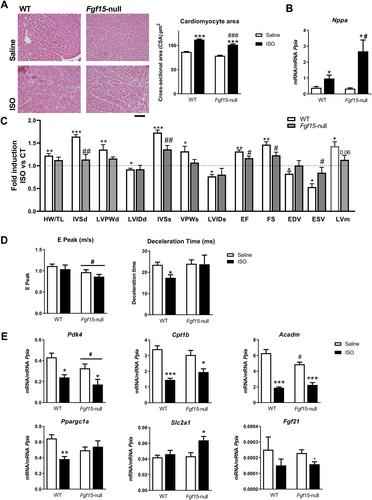
{"title":"一种新的FGF15/19介导的肠心轴控制心脏肥大","authors":"Samantha Morón-Ros, Albert Blasco-Roset, Artur Navarro-Gascon, Celia Rupérez, Monica Zamora, Fatima Crispi, Iker Uriarte, Maite G Fernández-Barrena, Matias Avila, Gemma Ferrer-Curriu, Josep Lupón, Antoni Bayés-Genis, Francesc Villarroya, Aleix Gavaldà-Navarro, Anna Planavila","doi":"10.1002/path.6193","DOIUrl":null,"url":null,"abstract":"<p>FGF15 and its human orthologue, FGF19, are members of the endocrine FGF family and are secreted by ileal enterocytes in response to bile acids. FGF15/19 mainly targets the liver, but recent studies indicate that it also regulates skeletal muscle mass and adipose tissue plasticity. The aim of this study was to determine the role(s) of the enterokine FGF15/19 during the development of cardiac hypertrophy. Studies in a cohort of humans suffering from heart failure showed increased circulating levels of FGF19 compared with control individuals. We found that mice lacking FGF15 did not develop cardiac hypertrophy in response to three different pathophysiological stimuli (high-fat diet, isoproterenol, or cold exposure). The heart weight/tibia length ratio and the cardiomyocyte area (as measures of cardiac hypertrophy development) under hypertrophy-inducing conditions were lower in <i>Fgf15</i>-null mice than in wild-type mice, whereas the levels of the cardiac damage marker atrial natriuretic factor (<i>Nppa</i>) were up-regulated. Echocardiographic measurements showed similar results. Moreover, the genes involved in fatty acid metabolism were down-regulated in <i>Fgf15</i>-null mice. Conversely, experimental increases in FGF15 induced cardiac hypertrophy <i>in vivo</i>, without changes in <i>Nppa</i> and up-regulation of metabolic genes. Finally, <i>in vitro</i> studies using cardiomyocytes showed that FGF19 had a direct effect on these cells promoting hypertrophy. We have identified herein an inter-organ signaling pathway that runs from the gut to the heart, acts through the enterokine FGF15/19, and is involved in cardiac hypertrophy development and regulation of fatty acid metabolism in the myocardium. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"261 3","pages":"335-348"},"PeriodicalIF":5.6000,"publicationDate":"2023-08-31","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6193","citationCount":"0","resultStr":"{\"title\":\"A new FGF15/19-mediated gut-to-heart axis controls cardiac hypertrophy\",\"authors\":\"Samantha Morón-Ros, Albert Blasco-Roset, Artur Navarro-Gascon, Celia Rupérez, Monica Zamora, Fatima Crispi, Iker Uriarte, Maite G Fernández-Barrena, Matias Avila, Gemma Ferrer-Curriu, Josep Lupón, Antoni Bayés-Genis, Francesc Villarroya, Aleix Gavaldà-Navarro, Anna Planavila\",\"doi\":\"10.1002/path.6193\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>FGF15 and its human orthologue, FGF19, are members of the endocrine FGF family and are secreted by ileal enterocytes in response to bile acids. FGF15/19 mainly targets the liver, but recent studies indicate that it also regulates skeletal muscle mass and adipose tissue plasticity. The aim of this study was to determine the role(s) of the enterokine FGF15/19 during the development of cardiac hypertrophy. Studies in a cohort of humans suffering from heart failure showed increased circulating levels of FGF19 compared with control individuals. We found that mice lacking FGF15 did not develop cardiac hypertrophy in response to three different pathophysiological stimuli (high-fat diet, isoproterenol, or cold exposure). The heart weight/tibia length ratio and the cardiomyocyte area (as measures of cardiac hypertrophy development) under hypertrophy-inducing conditions were lower in <i>Fgf15</i>-null mice than in wild-type mice, whereas the levels of the cardiac damage marker atrial natriuretic factor (<i>Nppa</i>) were up-regulated. Echocardiographic measurements showed similar results. Moreover, the genes involved in fatty acid metabolism were down-regulated in <i>Fgf15</i>-null mice. Conversely, experimental increases in FGF15 induced cardiac hypertrophy <i>in vivo</i>, without changes in <i>Nppa</i> and up-regulation of metabolic genes. Finally, <i>in vitro</i> studies using cardiomyocytes showed that FGF19 had a direct effect on these cells promoting hypertrophy. We have identified herein an inter-organ signaling pathway that runs from the gut to the heart, acts through the enterokine FGF15/19, and is involved in cardiac hypertrophy development and regulation of fatty acid metabolism in the myocardium. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"261 3\",\"pages\":\"335-348\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2023-08-31\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6193\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6193\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6193","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用