下载PDF
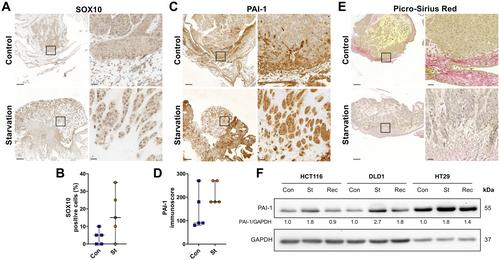
{"title":"在营养限制条件下存活的高侵袭性结直肠癌细胞的部分上皮-间充质转化特征。","authors":"Gil A Pastorino, Ilir Sheraj, Kerstin Huebner, Giulio Ferrero, Philipp Kunze, Arndt Hartmann, Chuanpit Hampel, Hepsen Hazal Husnugil, Arnatchai Maiuthed, Florian Gebhart, Fynn Schlattmann, Aliye Ezgi Gulec Taskiran, Goksu Oral, Ralph Palmisano, Barbara Pardini, Alessio Naccarati, Katharina Erlenbach-Wuensch, Sreeparna Banerjee, Regine Schneider-Stock","doi":"10.1002/path.6240","DOIUrl":null,"url":null,"abstract":"<p>Partial epithelial-mesenchymal transition (p-EMT) has recently been identified as a hybrid state consisting of cells with both epithelial and mesenchymal characteristics and is associated with the migration, metastasis, and chemoresistance of cancer cells. Here, we describe the induction of p-EMT in starved colorectal cancer (CRC) cells and identify a p-EMT gene signature that can predict prognosis. Functional characterisation of starvation-induced p-EMT in HCT116, DLD1, and HT29 cells showed changes in proliferation, morphology, and drug sensitivity, supported by <i>in vivo</i> studies using the chorioallantoic membrane model. An EMT-specific quantitative polymerase chain reaction (qPCR) array was used to screen for deregulated genes, leading to the establishment of an <i>in silico</i> gene signature that was correlated with poor disease-free survival in CRC patients along with the CRC consensus molecular subtype CMS4. Among the significantly deregulated p-EMT genes, a triple-gene signature consisting of <i>SERPINE1</i>, <i>SOX10</i>, and epidermal growth factor receptor (<i>EGFR</i>) was identified. Starvation-induced p-EMT was characterised by increased migratory potential and chemoresistance, as well as E-cadherin processing and internalisation. Both gene signature and E-cadherin alterations could be reversed by the proteasomal inhibitor MG132. Spatially resolving EGFR expression with high-resolution immunofluorescence imaging identified a proliferation stop in starved CRC cells caused by EGFR internalisation. In conclusion, we have gained insight into a previously undiscovered EMT mechanism that may become relevant when tumour cells are under nutrient stress, as seen in early stages of metastasis. Targeting this process of tumour cell dissemination might help to prevent EMT and overcome drug resistance. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"262 3","pages":"347-361"},"PeriodicalIF":5.6000,"publicationDate":"2024-01-18","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6240","citationCount":"0","resultStr":"{\"title\":\"A partial epithelial-mesenchymal transition signature for highly aggressive colorectal cancer cells that survive under nutrient restriction\",\"authors\":\"Gil A Pastorino, Ilir Sheraj, Kerstin Huebner, Giulio Ferrero, Philipp Kunze, Arndt Hartmann, Chuanpit Hampel, Hepsen Hazal Husnugil, Arnatchai Maiuthed, Florian Gebhart, Fynn Schlattmann, Aliye Ezgi Gulec Taskiran, Goksu Oral, Ralph Palmisano, Barbara Pardini, Alessio Naccarati, Katharina Erlenbach-Wuensch, Sreeparna Banerjee, Regine Schneider-Stock\",\"doi\":\"10.1002/path.6240\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Partial epithelial-mesenchymal transition (p-EMT) has recently been identified as a hybrid state consisting of cells with both epithelial and mesenchymal characteristics and is associated with the migration, metastasis, and chemoresistance of cancer cells. Here, we describe the induction of p-EMT in starved colorectal cancer (CRC) cells and identify a p-EMT gene signature that can predict prognosis. Functional characterisation of starvation-induced p-EMT in HCT116, DLD1, and HT29 cells showed changes in proliferation, morphology, and drug sensitivity, supported by <i>in vivo</i> studies using the chorioallantoic membrane model. An EMT-specific quantitative polymerase chain reaction (qPCR) array was used to screen for deregulated genes, leading to the establishment of an <i>in silico</i> gene signature that was correlated with poor disease-free survival in CRC patients along with the CRC consensus molecular subtype CMS4. Among the significantly deregulated p-EMT genes, a triple-gene signature consisting of <i>SERPINE1</i>, <i>SOX10</i>, and epidermal growth factor receptor (<i>EGFR</i>) was identified. Starvation-induced p-EMT was characterised by increased migratory potential and chemoresistance, as well as E-cadherin processing and internalisation. Both gene signature and E-cadherin alterations could be reversed by the proteasomal inhibitor MG132. Spatially resolving EGFR expression with high-resolution immunofluorescence imaging identified a proliferation stop in starved CRC cells caused by EGFR internalisation. In conclusion, we have gained insight into a previously undiscovered EMT mechanism that may become relevant when tumour cells are under nutrient stress, as seen in early stages of metastasis. Targeting this process of tumour cell dissemination might help to prevent EMT and overcome drug resistance. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"262 3\",\"pages\":\"347-361\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2024-01-18\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6240\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6240\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6240","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用