对鳞状细胞瘤进行详细的 DNA 甲基化分析,确定了恶性肿瘤的特征,并将鳞状细胞瘤与移行细胞乳腺癌区分开来。
Braydon Meyer, Clare Stirzaker, Sonny Ramkomuth, Kate Harvey, Belinda Chan, Cheok Soon Lee, Rooshdiya Karim, Niantao Deng, Kelly A Avery-Kiejda, Rodney J Scott, Sunil Lakhani, Stephen Fox, Elizabeth Robbins, Joo-Shik Shin, Jane Beith, Anthony Gill, Loretta Sioson, Charles Chan, Mrudula Krishnaswamy, Caroline Cooper, Sanjay Warrier, Cindy Mak, John EJ Rasko, Charles G Bailey, Alexander Swarbrick, Susan J Clark, Sandra O'Toole, Ruth Pidsley
下载PDF
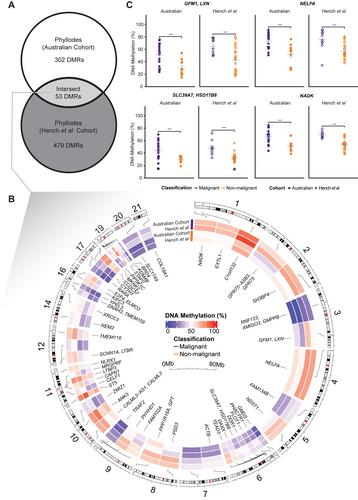
{"title":"对鳞状细胞瘤进行详细的 DNA 甲基化分析,确定了恶性肿瘤的特征,并将鳞状细胞瘤与移行细胞乳腺癌区分开来。","authors":"Braydon Meyer, Clare Stirzaker, Sonny Ramkomuth, Kate Harvey, Belinda Chan, Cheok Soon Lee, Rooshdiya Karim, Niantao Deng, Kelly A Avery-Kiejda, Rodney J Scott, Sunil Lakhani, Stephen Fox, Elizabeth Robbins, Joo-Shik Shin, Jane Beith, Anthony Gill, Loretta Sioson, Charles Chan, Mrudula Krishnaswamy, Caroline Cooper, Sanjay Warrier, Cindy Mak, John EJ Rasko, Charles G Bailey, Alexander Swarbrick, Susan J Clark, Sandra O'Toole, Ruth Pidsley","doi":"10.1002/path.6250","DOIUrl":null,"url":null,"abstract":"<p>Phyllodes tumours (PTs) are rare fibroepithelial lesions of the breast that are classified as benign, borderline, or malignant. As little is known about the molecular underpinnings of PTs, current diagnosis relies on histological examination. However, accurate classification is often difficult, particularly for distinguishing borderline from malignant PTs. Furthermore, PTs can be misdiagnosed as other tumour types with shared histological features, such as fibroadenoma and metaplastic breast cancers. As DNA methylation is a recognised hallmark of many cancers, we hypothesised that DNA methylation could provide novel biomarkers for diagnosis and tumour stratification in PTs, whilst also allowing insight into the molecular aetiology of this otherwise understudied tumour. We generated whole-genome methylation data using the Illumina EPIC microarray in a novel PT cohort (<i>n</i> = 33) and curated methylation microarray data from published datasets including PTs and other potentially histopathologically similar tumours (total <i>n</i> = 817 samples). Analyses revealed that PTs have a unique methylome compared to normal breast tissue and to potentially histopathologically similar tumours (metaplastic breast cancer, fibroadenoma and sarcomas), with PT-specific methylation changes enriched in gene sets involved in KRAS signalling and epithelial-mesenchymal transition. Next, we identified 53 differentially methylated regions (DMRs) (false discovery rate < 0.05) that specifically delineated malignant from non-malignant PTs. The top DMR in both discovery and validation cohorts was hypermethylation at the <i>HSD17B8</i> CpG island promoter. Matched PT single-cell expression data showed that <i>HSD17B8</i> had minimal expression in fibroblast (putative tumour) cells. Finally, we created a methylation classifier to distinguish PTs from metaplastic breast cancer samples, where we revealed a likely misdiagnosis for two TCGA metaplastic breast cancer samples. In conclusion, DNA methylation alterations are associated with PT histopathology and hold the potential to improve our understanding of PT molecular aetiology, diagnostics, and risk stratification. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"262 4","pages":"480-494"},"PeriodicalIF":5.6000,"publicationDate":"2024-02-01","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6250","citationCount":"0","resultStr":"{\"title\":\"Detailed DNA methylation characterisation of phyllodes tumours identifies a signature of malignancy and distinguishes phyllodes from metaplastic breast carcinoma\",\"authors\":\"Braydon Meyer, Clare Stirzaker, Sonny Ramkomuth, Kate Harvey, Belinda Chan, Cheok Soon Lee, Rooshdiya Karim, Niantao Deng, Kelly A Avery-Kiejda, Rodney J Scott, Sunil Lakhani, Stephen Fox, Elizabeth Robbins, Joo-Shik Shin, Jane Beith, Anthony Gill, Loretta Sioson, Charles Chan, Mrudula Krishnaswamy, Caroline Cooper, Sanjay Warrier, Cindy Mak, John EJ Rasko, Charles G Bailey, Alexander Swarbrick, Susan J Clark, Sandra O'Toole, Ruth Pidsley\",\"doi\":\"10.1002/path.6250\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Phyllodes tumours (PTs) are rare fibroepithelial lesions of the breast that are classified as benign, borderline, or malignant. As little is known about the molecular underpinnings of PTs, current diagnosis relies on histological examination. However, accurate classification is often difficult, particularly for distinguishing borderline from malignant PTs. Furthermore, PTs can be misdiagnosed as other tumour types with shared histological features, such as fibroadenoma and metaplastic breast cancers. As DNA methylation is a recognised hallmark of many cancers, we hypothesised that DNA methylation could provide novel biomarkers for diagnosis and tumour stratification in PTs, whilst also allowing insight into the molecular aetiology of this otherwise understudied tumour. We generated whole-genome methylation data using the Illumina EPIC microarray in a novel PT cohort (<i>n</i> = 33) and curated methylation microarray data from published datasets including PTs and other potentially histopathologically similar tumours (total <i>n</i> = 817 samples). Analyses revealed that PTs have a unique methylome compared to normal breast tissue and to potentially histopathologically similar tumours (metaplastic breast cancer, fibroadenoma and sarcomas), with PT-specific methylation changes enriched in gene sets involved in KRAS signalling and epithelial-mesenchymal transition. Next, we identified 53 differentially methylated regions (DMRs) (false discovery rate < 0.05) that specifically delineated malignant from non-malignant PTs. The top DMR in both discovery and validation cohorts was hypermethylation at the <i>HSD17B8</i> CpG island promoter. Matched PT single-cell expression data showed that <i>HSD17B8</i> had minimal expression in fibroblast (putative tumour) cells. Finally, we created a methylation classifier to distinguish PTs from metaplastic breast cancer samples, where we revealed a likely misdiagnosis for two TCGA metaplastic breast cancer samples. In conclusion, DNA methylation alterations are associated with PT histopathology and hold the potential to improve our understanding of PT molecular aetiology, diagnostics, and risk stratification. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"262 4\",\"pages\":\"480-494\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2024-02-01\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6250\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6250\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6250","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用