下载PDF
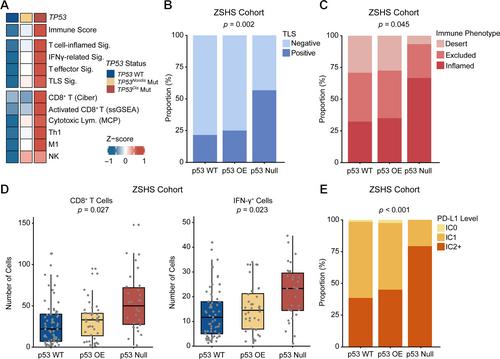
{"title":"TP53破坏性突变可预测铂类化疗和PD-1/PD-L1阻断剂对尿路上皮癌的反应。","authors":"Kaifeng Jin, Jingtong Xu, Xiaohe Su, Ziyue Xu, Bingyu Li, Ge Liu, Hailong Liu, Yiwei Wang, Yu Zhu, Le Xu, Weijuan Zhang, Zhaopei Liu, Zewei Wang, Yuan Chang, Jiejie Xu","doi":"10.1002/path.6266","DOIUrl":null,"url":null,"abstract":"<p><i>TP53</i> mutation is one of the most common genetic alterations in urothelial carcinoma (UrCa), and heterogeneity of <i>TP53</i> mutants leads to heterogeneous clinical outcomes. This study aimed to investigate the clinical relevance of specific <i>TP53</i> mutations in UrCa. In this study, a total of eight cohorts were enrolled, along with matched clinical annotation. <i>TP53</i> mutations were classified as disruptive and nondisruptive according to the degree of disturbance of p53 protein function and structure. We evaluated the clinical significance of <i>TP53</i> mutations in our local datasets and publicly available datasets. The co-occurring events of <i>TP53</i> mutations in UrCa, along with their therapeutic indications, functional effects, and the tumor immune microenvironment, were also investigated. <i>TP53</i> mutations were identified in 49.7% of the UrCa patients. Within this group, 25.1% of patients carried <i>TP53</i><sup>Disruptive</sup> mutations, a genetic alteration correlated with a significantly poorer overall survival (OS) when compared to individuals with <i>TP53</i><sup>Nondisruptive</sup> mutations and those with wild-type <i>TP53</i>. Significantly, patients with <i>TP53</i><sup>Disruptive</sup> mutations exhibit an increased probability of responding favorably to PD-1/PD-L1 blockade and chemoimmunotherapy. Meanwhile, there was no noteworthy distinction in OS among patients with varying <i>TP53</i> mutation status who underwent chemotherapy. Samples with <i>TP53</i><sup>Disruptive</sup> mutations showed an enriched APOBEC- and POLE-related mutational signature, as well as an elevated tumor mutation burden. The sensitivity to immunotherapy in tumors carrying <i>TP53</i><sup>Disruptive</sup> mutation may be attributed to the inflamed tumor microenvironment characterized by increased CD8<sup>+</sup>T cell infiltration and interferon-gamma signaling activation. In conclusion, UrCa patients with <i>TP53</i><sup>Disruptive</sup> mutations have shown reduced survival rates, yet they may respond well to PD-1/PD-L1 blockade therapy and chemoimmunotherapy. By distinguishing specific <i>TP53</i> mutations, we can improve risk stratification and offer personalized genomics-guided therapy to UrCa patients. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 2","pages":"139-149"},"PeriodicalIF":5.6000,"publicationDate":"2024-02-21","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6266","citationCount":"0","resultStr":"{\"title\":\"TP53 disruptive mutation predicts platinum-based chemotherapy and PD-1/PD-L1 blockade response in urothelial carcinoma\",\"authors\":\"Kaifeng Jin, Jingtong Xu, Xiaohe Su, Ziyue Xu, Bingyu Li, Ge Liu, Hailong Liu, Yiwei Wang, Yu Zhu, Le Xu, Weijuan Zhang, Zhaopei Liu, Zewei Wang, Yuan Chang, Jiejie Xu\",\"doi\":\"10.1002/path.6266\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p><i>TP53</i> mutation is one of the most common genetic alterations in urothelial carcinoma (UrCa), and heterogeneity of <i>TP53</i> mutants leads to heterogeneous clinical outcomes. This study aimed to investigate the clinical relevance of specific <i>TP53</i> mutations in UrCa. In this study, a total of eight cohorts were enrolled, along with matched clinical annotation. <i>TP53</i> mutations were classified as disruptive and nondisruptive according to the degree of disturbance of p53 protein function and structure. We evaluated the clinical significance of <i>TP53</i> mutations in our local datasets and publicly available datasets. The co-occurring events of <i>TP53</i> mutations in UrCa, along with their therapeutic indications, functional effects, and the tumor immune microenvironment, were also investigated. <i>TP53</i> mutations were identified in 49.7% of the UrCa patients. Within this group, 25.1% of patients carried <i>TP53</i><sup>Disruptive</sup> mutations, a genetic alteration correlated with a significantly poorer overall survival (OS) when compared to individuals with <i>TP53</i><sup>Nondisruptive</sup> mutations and those with wild-type <i>TP53</i>. Significantly, patients with <i>TP53</i><sup>Disruptive</sup> mutations exhibit an increased probability of responding favorably to PD-1/PD-L1 blockade and chemoimmunotherapy. Meanwhile, there was no noteworthy distinction in OS among patients with varying <i>TP53</i> mutation status who underwent chemotherapy. Samples with <i>TP53</i><sup>Disruptive</sup> mutations showed an enriched APOBEC- and POLE-related mutational signature, as well as an elevated tumor mutation burden. The sensitivity to immunotherapy in tumors carrying <i>TP53</i><sup>Disruptive</sup> mutation may be attributed to the inflamed tumor microenvironment characterized by increased CD8<sup>+</sup>T cell infiltration and interferon-gamma signaling activation. In conclusion, UrCa patients with <i>TP53</i><sup>Disruptive</sup> mutations have shown reduced survival rates, yet they may respond well to PD-1/PD-L1 blockade therapy and chemoimmunotherapy. By distinguishing specific <i>TP53</i> mutations, we can improve risk stratification and offer personalized genomics-guided therapy to UrCa patients. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"263 2\",\"pages\":\"139-149\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2024-02-21\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6266\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6266\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6266","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用