下载PDF
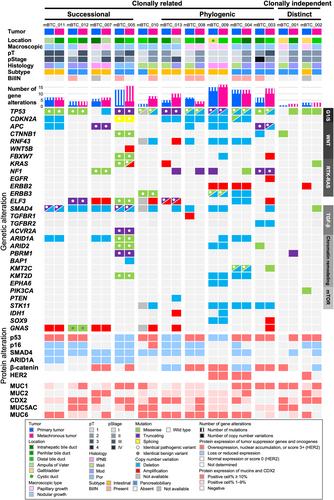
{"title":"变异双胆道癌的克隆分析","authors":"Yuko Omori, Shuichi Aoki, Yusuke Ono, Takashi Kokumai, Shingo Yoshimachi, Hideaki Sato, Akiko Kusaka, Masahiro Iseki, Daisuke Douchi, Takayuki Miura, Shimpei Maeda, Masaharu Ishida, Masamichi Mizuma, Kei Nakagawa, Yusuke Mizukami, Toru Furukawa, Michiaki Unno","doi":"10.1002/path.6265","DOIUrl":null,"url":null,"abstract":"<p>The molecular mechanisms underpinning the development of metachronous tumors in the remnant bile duct following surgical resection of primary biliary tract carcinomas (BTCs) are unknown. This study aimed to elucidate these mechanisms by evaluating the clinicopathologic features of BTCs, the alterations to 31 BTC-related genes on targeted sequencing, and the aberrant expression of p53, p16, SMAD4, ARID1A and β-catenin on immunohistochemistry. Twelve consecutive patients who underwent resection of metachronous BTCs following primary BTC resection with negative bile duct margins were enrolled. Among the 12 metachronous tumors, six exhibited anterograde growth in the lower portion and six exhibited retrograde growth in the upper portion of the biliary tree. Surgical resection of metachronous BTCs resulted in recurrence-free survival in seven, local recurrence in five, and death in two patients. Nine achieved 5-year overall survival after primary surgery. Molecular analyses revealed that recurrently altered genes were: <i>TP53</i>, <i>SMAD4</i>, <i>CDKN2A</i>, <i>ELF3</i>, <i>ARID1A</i>, <i>GNAS</i>, <i>NF1</i>, <i>STK11</i>, <i>RNF43</i>, <i>KMT2D</i> and <i>ERBB3</i>. Each of these was altered in at least three cases. A comparison of the molecular features between 12 paired primary and metachronous BTCs indicated that 10 (83%) metachronous tumors developed in clonal association with corresponding primary tumors either successionally or phylogenically. The remaining two (17%) developed distinctly. The successional tumors consisted of direct or evolved primary tumor clones that spread along the bile duct. The phylogenic tumors consisted of genetically unstable clones and conferred a poor prognosis. Metachronous tumors distinct from their primaries harbored fewer mutations than successional and phylogenic tumors. In conclusion, over 80% of metachronous BTCs that develop following primary BTC resection are probably molecularly associated with their primaries in either a successional or a phylogenetic manner. Comparison between the molecular features of a metachronous tumor and those of a preceding tumor may provide effective therapeutic clues for the treatment of metachronous BTC. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 1","pages":"113-127"},"PeriodicalIF":5.6000,"publicationDate":"2024-03-14","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6265","citationCount":"0","resultStr":"{\"title\":\"Clonal analysis of metachronous double biliary tract cancers\",\"authors\":\"Yuko Omori, Shuichi Aoki, Yusuke Ono, Takashi Kokumai, Shingo Yoshimachi, Hideaki Sato, Akiko Kusaka, Masahiro Iseki, Daisuke Douchi, Takayuki Miura, Shimpei Maeda, Masaharu Ishida, Masamichi Mizuma, Kei Nakagawa, Yusuke Mizukami, Toru Furukawa, Michiaki Unno\",\"doi\":\"10.1002/path.6265\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>The molecular mechanisms underpinning the development of metachronous tumors in the remnant bile duct following surgical resection of primary biliary tract carcinomas (BTCs) are unknown. This study aimed to elucidate these mechanisms by evaluating the clinicopathologic features of BTCs, the alterations to 31 BTC-related genes on targeted sequencing, and the aberrant expression of p53, p16, SMAD4, ARID1A and β-catenin on immunohistochemistry. Twelve consecutive patients who underwent resection of metachronous BTCs following primary BTC resection with negative bile duct margins were enrolled. Among the 12 metachronous tumors, six exhibited anterograde growth in the lower portion and six exhibited retrograde growth in the upper portion of the biliary tree. Surgical resection of metachronous BTCs resulted in recurrence-free survival in seven, local recurrence in five, and death in two patients. Nine achieved 5-year overall survival after primary surgery. Molecular analyses revealed that recurrently altered genes were: <i>TP53</i>, <i>SMAD4</i>, <i>CDKN2A</i>, <i>ELF3</i>, <i>ARID1A</i>, <i>GNAS</i>, <i>NF1</i>, <i>STK11</i>, <i>RNF43</i>, <i>KMT2D</i> and <i>ERBB3</i>. Each of these was altered in at least three cases. A comparison of the molecular features between 12 paired primary and metachronous BTCs indicated that 10 (83%) metachronous tumors developed in clonal association with corresponding primary tumors either successionally or phylogenically. The remaining two (17%) developed distinctly. The successional tumors consisted of direct or evolved primary tumor clones that spread along the bile duct. The phylogenic tumors consisted of genetically unstable clones and conferred a poor prognosis. Metachronous tumors distinct from their primaries harbored fewer mutations than successional and phylogenic tumors. In conclusion, over 80% of metachronous BTCs that develop following primary BTC resection are probably molecularly associated with their primaries in either a successional or a phylogenetic manner. Comparison between the molecular features of a metachronous tumor and those of a preceding tumor may provide effective therapeutic clues for the treatment of metachronous BTC. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"263 1\",\"pages\":\"113-127\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2024-03-14\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6265\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6265\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6265","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用