下载PDF
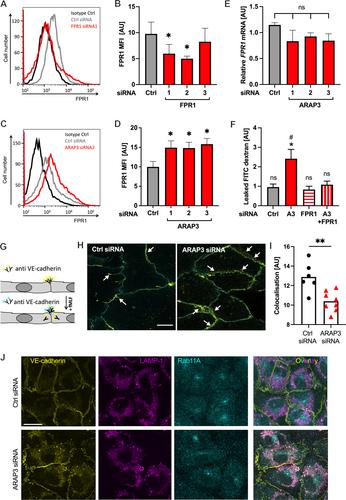
{"title":"ARAP3 通过作用于内皮细胞和中性粒细胞,防止过度甲酰肽诱导的微血管渗漏。","authors":"Julia Y Chu, Barry McCormick, Kruthika Sundaram, Gareth Hardisty, Utsa Karmakar, Caroline Pumpe, Elizabeth Krull, Christopher D Lucas, Joana Amado-Azevedo, Peter L Hordijk, Andrea Caporali, Harry Mellor, J Kenneth Baillie, Adriano G Rossi, Sonja Vermeren","doi":"10.1002/path.6288","DOIUrl":null,"url":null,"abstract":"<p>Vascular permeability is temporarily heightened during inflammation, but excessive inflammation-associated microvascular leakage can be detrimental, as evidenced in the inflamed lung. Formylated peptides regulate vascular leakage indirectly via formylated peptide receptor-1 (FPR1)-mediated recruitment and activation of neutrophils. Here we identify how the GTPase-activating protein ARAP3 protects against formylated peptide-induced microvascular permeability via endothelial cells and neutrophils. <i>In vitro</i>, <i>Arap3</i><sup>−/−</sup> endothelial monolayers were characterised by enhanced formylated peptide-induced permeability due to upregulated endothelial FPR1 and enhanced vascular endothelial cadherin internalisation. <i>In vivo</i>, enhanced inflammation-associated microvascular leakage was observed in <i>Arap3</i><sup>−/−</sup> mice. Leakage of plasma protein into the lungs of <i>Arap3</i><sup>−/−</sup> mice increased within hours of formylated peptide administration. Adoptive transfer experiments indicated this was dependent upon ARAP3 deficiency in both immune and non-immune cells. Bronchoalveolar lavages of formylated peptide-challenged <i>Arap3</i><sup>−/−</sup> mice contained neutrophil extracellular traps (NETs). Pharmacological inhibition of NET formation abrogated excessive microvascular leakage, indicating a critical function of NETs in this context. The observation that <i>Arap3</i><sup>−/−</sup> mice developed more severe influenza suggests these findings are pertinent to pathological situations characterised by abundant formylated peptides. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 3","pages":"347-359"},"PeriodicalIF":5.6000,"publicationDate":"2024-05-11","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6288","citationCount":"0","resultStr":"{\"title\":\"ARAP3 protects from excessive formylated peptide-induced microvascular leakage by acting on endothelial cells and neutrophils\",\"authors\":\"Julia Y Chu, Barry McCormick, Kruthika Sundaram, Gareth Hardisty, Utsa Karmakar, Caroline Pumpe, Elizabeth Krull, Christopher D Lucas, Joana Amado-Azevedo, Peter L Hordijk, Andrea Caporali, Harry Mellor, J Kenneth Baillie, Adriano G Rossi, Sonja Vermeren\",\"doi\":\"10.1002/path.6288\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Vascular permeability is temporarily heightened during inflammation, but excessive inflammation-associated microvascular leakage can be detrimental, as evidenced in the inflamed lung. Formylated peptides regulate vascular leakage indirectly via formylated peptide receptor-1 (FPR1)-mediated recruitment and activation of neutrophils. Here we identify how the GTPase-activating protein ARAP3 protects against formylated peptide-induced microvascular permeability via endothelial cells and neutrophils. <i>In vitro</i>, <i>Arap3</i><sup>−/−</sup> endothelial monolayers were characterised by enhanced formylated peptide-induced permeability due to upregulated endothelial FPR1 and enhanced vascular endothelial cadherin internalisation. <i>In vivo</i>, enhanced inflammation-associated microvascular leakage was observed in <i>Arap3</i><sup>−/−</sup> mice. Leakage of plasma protein into the lungs of <i>Arap3</i><sup>−/−</sup> mice increased within hours of formylated peptide administration. Adoptive transfer experiments indicated this was dependent upon ARAP3 deficiency in both immune and non-immune cells. Bronchoalveolar lavages of formylated peptide-challenged <i>Arap3</i><sup>−/−</sup> mice contained neutrophil extracellular traps (NETs). Pharmacological inhibition of NET formation abrogated excessive microvascular leakage, indicating a critical function of NETs in this context. The observation that <i>Arap3</i><sup>−/−</sup> mice developed more severe influenza suggests these findings are pertinent to pathological situations characterised by abundant formylated peptides. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"263 3\",\"pages\":\"347-359\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2024-05-11\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6288\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6288\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6288","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用