{"title":"利用哺乳动物细胞裂解液进行微管相互作用蛋白中通量无细胞分析的平台","authors":"A. S. Jijumon, Arya Krishnan, Carsten Janke","doi":"10.1002/cpz1.1070","DOIUrl":null,"url":null,"abstract":"<p>The microtubule (MT) cytoskeleton performs a variety of functions in cell division, cell architecture, neuronal differentiation, and ciliary beating. These functions are controlled by proteins that directly interact with MTs, commonly referred to as microtubule-associated proteins (MAPs). Out of the many proteins reported interact with MTs, only a some have been biochemically and functionally characterized so far. One of the limitations of classical in vitro assays and single-MT reconstitution approaches is that they are typically performed with purified proteins. As purification of proteins can be difficult and time-consuming, many previous studies have only focused on a few proteins, while systematic analyses of many different proteins by in vitro reconstitution assays were not possible. Here we present a detailed protocol using lysates of mammalian cells instead of purified proteins that overcomes this limitation. Those lysates contain all molecular components required for in vitro MT reconstitution including the endogenous tubulin and the recombinant MAPs, which form MT assemblies upon the injection of the lysates into a microscopy chamber. This allows to directly observe the dynamic behavior of growing MTs, as well as the fluorescently labeled associated proteins by total internal reflection fluorescence (TIRF) microscopy. Strikingly, all proteins tested so far were functional in our approach, thus providing the possibility to test virtually any protein of interest. This also opens the possibility to screen the impact of patient mutations on the MT binding behavior of MAPs in a medium-throughput manner. In addition, the lysate approach can easily be adapted to other applications that have predominantly been performed with purified proteins so far, such as investigating other cytoskeletal systems and cytoskeletal crosstalk, or to study structures of MAPs bound to MTs by cryo-electron microscopy. Our approach is thus a versatile, expandable, and easy-to-use method to characterize the impact of a broad spectrum of proteins on cytoskeletal behavior and function. © 2024 The Authors. Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Preparation of lysates of human cells for TIRF reconstitution assays</p><p><b>Basic Protocol 2</b>: Quantification of GFP-tagged MAP concentration in cell lysates</p><p><b>Support Protocol 1</b>: Purification of KIF5B(N555/T92A) (dead kinesin) protein for TIRF reconstitution assays</p><p><b>Support Protocol 2</b>: Preparation of GMPCPP MT seeds for TIRF reconstitution assays</p><p><b>Basic Protocol 3</b>: TIRF-based MT-MAP reconstitution assays using cell lysates</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":null,"pages":null},"PeriodicalIF":0.0000,"publicationDate":"2024-06-12","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.1070","citationCount":"0","resultStr":"{\"title\":\"A Platform for Medium-Throughput Cell-Free Analyses of Microtubule-Interacting Proteins Using Mammalian Cell Lysates\",\"authors\":\"A. S. Jijumon, Arya Krishnan, Carsten Janke\",\"doi\":\"10.1002/cpz1.1070\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>The microtubule (MT) cytoskeleton performs a variety of functions in cell division, cell architecture, neuronal differentiation, and ciliary beating. These functions are controlled by proteins that directly interact with MTs, commonly referred to as microtubule-associated proteins (MAPs). Out of the many proteins reported interact with MTs, only a some have been biochemically and functionally characterized so far. One of the limitations of classical in vitro assays and single-MT reconstitution approaches is that they are typically performed with purified proteins. As purification of proteins can be difficult and time-consuming, many previous studies have only focused on a few proteins, while systematic analyses of many different proteins by in vitro reconstitution assays were not possible. Here we present a detailed protocol using lysates of mammalian cells instead of purified proteins that overcomes this limitation. Those lysates contain all molecular components required for in vitro MT reconstitution including the endogenous tubulin and the recombinant MAPs, which form MT assemblies upon the injection of the lysates into a microscopy chamber. This allows to directly observe the dynamic behavior of growing MTs, as well as the fluorescently labeled associated proteins by total internal reflection fluorescence (TIRF) microscopy. Strikingly, all proteins tested so far were functional in our approach, thus providing the possibility to test virtually any protein of interest. This also opens the possibility to screen the impact of patient mutations on the MT binding behavior of MAPs in a medium-throughput manner. In addition, the lysate approach can easily be adapted to other applications that have predominantly been performed with purified proteins so far, such as investigating other cytoskeletal systems and cytoskeletal crosstalk, or to study structures of MAPs bound to MTs by cryo-electron microscopy. Our approach is thus a versatile, expandable, and easy-to-use method to characterize the impact of a broad spectrum of proteins on cytoskeletal behavior and function. © 2024 The Authors. Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Preparation of lysates of human cells for TIRF reconstitution assays</p><p><b>Basic Protocol 2</b>: Quantification of GFP-tagged MAP concentration in cell lysates</p><p><b>Support Protocol 1</b>: Purification of KIF5B(N555/T92A) (dead kinesin) protein for TIRF reconstitution assays</p><p><b>Support Protocol 2</b>: Preparation of GMPCPP MT seeds for TIRF reconstitution assays</p><p><b>Basic Protocol 3</b>: TIRF-based MT-MAP reconstitution assays using cell lysates</p>\",\"PeriodicalId\":93970,\"journal\":{\"name\":\"Current protocols\",\"volume\":null,\"pages\":null},\"PeriodicalIF\":0.0000,\"publicationDate\":\"2024-06-12\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.1070\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Current protocols\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/cpz1.1070\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"\",\"JCRName\":\"\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpz1.1070","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
A Platform for Medium-Throughput Cell-Free Analyses of Microtubule-Interacting Proteins Using Mammalian Cell Lysates
The microtubule (MT) cytoskeleton performs a variety of functions in cell division, cell architecture, neuronal differentiation, and ciliary beating. These functions are controlled by proteins that directly interact with MTs, commonly referred to as microtubule-associated proteins (MAPs). Out of the many proteins reported interact with MTs, only a some have been biochemically and functionally characterized so far. One of the limitations of classical in vitro assays and single-MT reconstitution approaches is that they are typically performed with purified proteins. As purification of proteins can be difficult and time-consuming, many previous studies have only focused on a few proteins, while systematic analyses of many different proteins by in vitro reconstitution assays were not possible. Here we present a detailed protocol using lysates of mammalian cells instead of purified proteins that overcomes this limitation. Those lysates contain all molecular components required for in vitro MT reconstitution including the endogenous tubulin and the recombinant MAPs, which form MT assemblies upon the injection of the lysates into a microscopy chamber. This allows to directly observe the dynamic behavior of growing MTs, as well as the fluorescently labeled associated proteins by total internal reflection fluorescence (TIRF) microscopy. Strikingly, all proteins tested so far were functional in our approach, thus providing the possibility to test virtually any protein of interest. This also opens the possibility to screen the impact of patient mutations on the MT binding behavior of MAPs in a medium-throughput manner. In addition, the lysate approach can easily be adapted to other applications that have predominantly been performed with purified proteins so far, such as investigating other cytoskeletal systems and cytoskeletal crosstalk, or to study structures of MAPs bound to MTs by cryo-electron microscopy. Our approach is thus a versatile, expandable, and easy-to-use method to characterize the impact of a broad spectrum of proteins on cytoskeletal behavior and function. © 2024 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1: Preparation of lysates of human cells for TIRF reconstitution assays
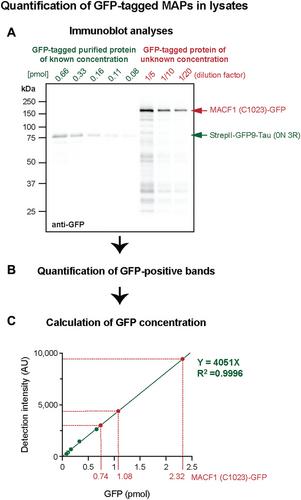
Basic Protocol 2: Quantification of GFP-tagged MAP concentration in cell lysates
Support Protocol 1: Purification of KIF5B(N555/T92A) (dead kinesin) protein for TIRF reconstitution assays
Support Protocol 2: Preparation of GMPCPP MT seeds for TIRF reconstitution assays
Basic Protocol 3: TIRF-based MT-MAP reconstitution assays using cell lysates