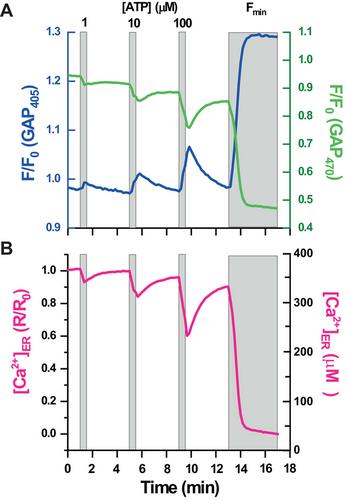
{"title":"使用荧光 GAP 指示剂监测 ER Ca2。","authors":"Jonathan Rojo-Ruiz, Cinthia Sánchez-Rabadán, Belen Calvo, Javier García-Sancho, Maria Teresa Alonso","doi":"10.1002/cpz1.1060","DOIUrl":null,"url":null,"abstract":"<p>The endoplasmic reticulum (ER) is the main reservoir of Ca<sup>2+</sup> of the cell. Accurate and quantitative measuring of Ca<sup>2+</sup> dynamics within the lumen of the ER has been challenging. In the last decade a few genetically encoded Ca<sup>2+</sup> indicators have been developed, including a family of fluorescent Ca<sup>2+</sup> indicators, dubbed GFP-Aequorin Proteins (GAPs). They are based on the fusion of two jellyfish proteins, the green fluorescent protein (GFP) and the Ca<sup>2+</sup>-binding protein aequorin. GAP Ca<sup>2+</sup> indicators exhibit a combination of several features: they are excitation ratiometric indicators, with reciprocal changes in the fluorescence excited at 405 and 470 nm, which is advantageous for imaging experiments; they exhibit a Hill coefficient of 1, which facilitates the calibration of the fluorescent signal into Ca<sup>2+</sup> concentrations; they are insensible to variations in the Mg<sup>2+</sup> concentrations or pH variations (in the 6.5-8.5 range); and, due to the lack of mammalian homologues, these proteins have a favorable expression in transgenic animals. A low Ca<sup>2+</sup> affinity version of GAP, GAP3 (K<sub>D</sub> ≅ 489 µM), has been engineered to conform with the estimated [Ca<sup>2+</sup>] in the ER. GAP3 targeted to the lumen of the ER (erGAP3) can be utilized for imaging intraluminal Ca<sup>2+</sup>. The ratiometric measurements provide a quantitative method to assess accurate [Ca<sup>2+</sup>]<sub>ER</sub>, both dynamically and at rest. In addition, erGAP3 can be combined with synthetic cytosolic Ca<sup>2+</sup> indicators to simultaneously monitor ER and cytosolic Ca<sup>2+</sup>. Here, we provide detailed methods to assess erGAP3 expression and to perform Ca<sup>2+</sup> imaging, either restricted to the ER lumen, or simultaneously in the ER and the cytosol. © 2024 The Authors. Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Detection of erGAP3 in the ER by immunofluorescence</p><p><b>Basic Protocol 2</b>: Monitoring ER Ca<sup>2+</sup></p><p><b>Basic Protocol 3</b>: Monitoring ER- and cytosolic-Ca<sup>2+</sup></p><p><b>Support Protocol</b>: Generation of a stable cell line expressing erGAP3</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":null,"pages":null},"PeriodicalIF":0.0000,"publicationDate":"2024-06-24","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.1060","citationCount":"0","resultStr":"{\"title\":\"Using Fluorescent GAP Indicators to Monitor ER Ca2+\",\"authors\":\"Jonathan Rojo-Ruiz, Cinthia Sánchez-Rabadán, Belen Calvo, Javier García-Sancho, Maria Teresa Alonso\",\"doi\":\"10.1002/cpz1.1060\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>The endoplasmic reticulum (ER) is the main reservoir of Ca<sup>2+</sup> of the cell. Accurate and quantitative measuring of Ca<sup>2+</sup> dynamics within the lumen of the ER has been challenging. In the last decade a few genetically encoded Ca<sup>2+</sup> indicators have been developed, including a family of fluorescent Ca<sup>2+</sup> indicators, dubbed GFP-Aequorin Proteins (GAPs). They are based on the fusion of two jellyfish proteins, the green fluorescent protein (GFP) and the Ca<sup>2+</sup>-binding protein aequorin. GAP Ca<sup>2+</sup> indicators exhibit a combination of several features: they are excitation ratiometric indicators, with reciprocal changes in the fluorescence excited at 405 and 470 nm, which is advantageous for imaging experiments; they exhibit a Hill coefficient of 1, which facilitates the calibration of the fluorescent signal into Ca<sup>2+</sup> concentrations; they are insensible to variations in the Mg<sup>2+</sup> concentrations or pH variations (in the 6.5-8.5 range); and, due to the lack of mammalian homologues, these proteins have a favorable expression in transgenic animals. A low Ca<sup>2+</sup> affinity version of GAP, GAP3 (K<sub>D</sub> ≅ 489 µM), has been engineered to conform with the estimated [Ca<sup>2+</sup>] in the ER. GAP3 targeted to the lumen of the ER (erGAP3) can be utilized for imaging intraluminal Ca<sup>2+</sup>. The ratiometric measurements provide a quantitative method to assess accurate [Ca<sup>2+</sup>]<sub>ER</sub>, both dynamically and at rest. In addition, erGAP3 can be combined with synthetic cytosolic Ca<sup>2+</sup> indicators to simultaneously monitor ER and cytosolic Ca<sup>2+</sup>. Here, we provide detailed methods to assess erGAP3 expression and to perform Ca<sup>2+</sup> imaging, either restricted to the ER lumen, or simultaneously in the ER and the cytosol. © 2024 The Authors. Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Detection of erGAP3 in the ER by immunofluorescence</p><p><b>Basic Protocol 2</b>: Monitoring ER Ca<sup>2+</sup></p><p><b>Basic Protocol 3</b>: Monitoring ER- and cytosolic-Ca<sup>2+</sup></p><p><b>Support Protocol</b>: Generation of a stable cell line expressing erGAP3</p>\",\"PeriodicalId\":93970,\"journal\":{\"name\":\"Current protocols\",\"volume\":null,\"pages\":null},\"PeriodicalIF\":0.0000,\"publicationDate\":\"2024-06-24\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.1060\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Current protocols\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/cpz1.1060\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"\",\"JCRName\":\"\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpz1.1060","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0