求助PDF
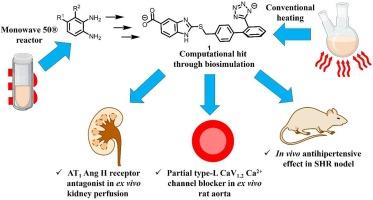
{"title":"具有抗高血压多靶点效应的苯并咪唑衍生物的合成、生物模拟和药理学评价。","authors":"Abraham Gutiérrez-Hernández , Samuel Estrada-Soto , Carlos Martínez-Conde , Emmanuel Gaona-Tovar , José L. Medina-Franco , Emanuel Hernández-Núñez , Sergio Hidalgo-Figueroa , Patricia Castro-Moreno , Maximiliano Ibarra-Barajas , Gabriel Navarrete-Vazquez","doi":"10.1016/j.bmcl.2024.129879","DOIUrl":null,"url":null,"abstract":"<div><p>In this study, we synthesized a series of seven benzimidazole derivatives incorporating the structural acidic framework of angiotensin II (Ang II) type 1 receptor (AT<sub>1</sub>R) antagonists (ARA-II) employing a three-step reaction sequence. The chemical structures were confirmed by <sup>1</sup>H NMR, <sup>13</sup>C NMR and mass spectral data. Through biosimulation, compounds <strong>1</strong>–<strong>7</strong> were identified as computational safe hits, thus, best candidates underwent <em>ex vivo</em> testing against two distinct mechanisms implicated in hypertension: antagonism of the Ang II type 1 receptor and the blockade of calcium channel. Molecular docking studies helped to understand at the molecular level the dual vasorelaxant effects with the recognition sites of the AT<sub>1</sub>R and the L-type calcium channel. In an <em>in vivo</em> spontaneously hypertensive rat model (SHR), intraperitoneally administration of compound <strong>1</strong> at 20 mg/kg resulted in a 25 % reduction in systolic blood pressure, demonstrating both <em>ex vivo</em> vasorelaxant action and <em>in vivo</em> antihypertensive multitarget efficacy. ©2024 Elsevier.</p></div>","PeriodicalId":256,"journal":{"name":"Bioorganic & Medicinal Chemistry Letters","volume":null,"pages":null},"PeriodicalIF":2.5000,"publicationDate":"2024-07-06","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"","citationCount":"0","resultStr":"{\"title\":\"Synthesis, biosimulation and pharmacological evaluation of benzimidazole derivatives with antihypertensive multitarget effect\",\"authors\":\"Abraham Gutiérrez-Hernández , Samuel Estrada-Soto , Carlos Martínez-Conde , Emmanuel Gaona-Tovar , José L. Medina-Franco , Emanuel Hernández-Núñez , Sergio Hidalgo-Figueroa , Patricia Castro-Moreno , Maximiliano Ibarra-Barajas , Gabriel Navarrete-Vazquez\",\"doi\":\"10.1016/j.bmcl.2024.129879\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<div><p>In this study, we synthesized a series of seven benzimidazole derivatives incorporating the structural acidic framework of angiotensin II (Ang II) type 1 receptor (AT<sub>1</sub>R) antagonists (ARA-II) employing a three-step reaction sequence. The chemical structures were confirmed by <sup>1</sup>H NMR, <sup>13</sup>C NMR and mass spectral data. Through biosimulation, compounds <strong>1</strong>–<strong>7</strong> were identified as computational safe hits, thus, best candidates underwent <em>ex vivo</em> testing against two distinct mechanisms implicated in hypertension: antagonism of the Ang II type 1 receptor and the blockade of calcium channel. Molecular docking studies helped to understand at the molecular level the dual vasorelaxant effects with the recognition sites of the AT<sub>1</sub>R and the L-type calcium channel. In an <em>in vivo</em> spontaneously hypertensive rat model (SHR), intraperitoneally administration of compound <strong>1</strong> at 20 mg/kg resulted in a 25 % reduction in systolic blood pressure, demonstrating both <em>ex vivo</em> vasorelaxant action and <em>in vivo</em> antihypertensive multitarget efficacy. ©2024 Elsevier.</p></div>\",\"PeriodicalId\":256,\"journal\":{\"name\":\"Bioorganic & Medicinal Chemistry Letters\",\"volume\":null,\"pages\":null},\"PeriodicalIF\":2.5000,\"publicationDate\":\"2024-07-06\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Bioorganic & Medicinal Chemistry Letters\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://www.sciencedirect.com/science/article/pii/S0960894X24002816\",\"RegionNum\":4,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q3\",\"JCRName\":\"CHEMISTRY, MEDICINAL\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Bioorganic & Medicinal Chemistry Letters","FirstCategoryId":"3","ListUrlMain":"https://www.sciencedirect.com/science/article/pii/S0960894X24002816","RegionNum":4,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q3","JCRName":"CHEMISTRY, MEDICINAL","Score":null,"Total":0}
引用次数: 0
引用
批量引用