{"title":"定义泛凋亡:先天性免疫细胞死亡激活的生化和机制评估。","authors":"Rebecca E. Tweedell, Taylor Hibler, Thirumala-Devi Kanneganti","doi":"10.1002/cpz1.1112","DOIUrl":null,"url":null,"abstract":"<p>The innate immune system is the first line of host defense. Innate immune activation utilizes pattern recognition receptors to detect pathogens, pathogen-associated and damage-associated molecular patterns (PAMPs and DAMPs), and homeostatic alterations and drives inflammatory signaling pathways and regulated cell death. Cell death activation is critical to eliminate pathogens and aberrant or damaged cells, while excess activation can be linked to inflammation, tissue damage, and disease. Therefore, there is increasing interest in studying cell death mechanisms to understand the underlying biology and identify therapeutic strategies. However, there are significant technical challenges, as many cell death pathways share key molecules with each other, and genetic models where these cell death molecules are deleted remain the gold standard for evaluation. Furthermore, extensive crosstalk has been identified between the cell death pathways pyroptosis, apoptosis, necroptosis, and the more recently characterized PANoptosis, which is defined as a prominent, unique innate immune, lytic, and inflammatory cell death pathway initiated by innate immune sensors and driven by caspases and RIPKs through PANoptosomes. PANoptosomes are multi-protein complexes assembled by innate immune sensor(s) in response to pathogens, PAMPs, DAMPs, cytokines, and homeostatic changes that drive PANoptosis. In this article, we provide methods for molecularly defining distinct cell death pathways, including PANoptosis, using both genetic and chemical approaches through western blot, LDH assay, and microscopy readouts. This procedure allows for the assessment of cell death on the cell population and single-cell levels even without access to genetic models. Having this comprehensive workflow that is more accessible to all labs will improve our ability as a scientific community to accelerate discovery. Using these protocols will help identify new innate immune sensors that drive PANoptosis and define the molecular mechanisms and regulators involved to establish new targets for clinical translation. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Induction and quantification of cell death using live cell imaging</p><p><b>Alternate Protocol 1</b>: Quantification of cell death using LDH</p><p><b>Alternate Protocol 2</b>: Assessment of cell death complexes in single cells using immunofluorescence staining</p><p><b>Basic Protocol 2</b>: Analysis of cell death mechanisms by immunoblots (western blots)</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"4 7","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2024-07-29","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.1112","citationCount":"0","resultStr":"{\"title\":\"Defining PANoptosis: Biochemical and Mechanistic Evaluation of Innate Immune Cell Death Activation\",\"authors\":\"Rebecca E. Tweedell, Taylor Hibler, Thirumala-Devi Kanneganti\",\"doi\":\"10.1002/cpz1.1112\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>The innate immune system is the first line of host defense. Innate immune activation utilizes pattern recognition receptors to detect pathogens, pathogen-associated and damage-associated molecular patterns (PAMPs and DAMPs), and homeostatic alterations and drives inflammatory signaling pathways and regulated cell death. Cell death activation is critical to eliminate pathogens and aberrant or damaged cells, while excess activation can be linked to inflammation, tissue damage, and disease. Therefore, there is increasing interest in studying cell death mechanisms to understand the underlying biology and identify therapeutic strategies. However, there are significant technical challenges, as many cell death pathways share key molecules with each other, and genetic models where these cell death molecules are deleted remain the gold standard for evaluation. Furthermore, extensive crosstalk has been identified between the cell death pathways pyroptosis, apoptosis, necroptosis, and the more recently characterized PANoptosis, which is defined as a prominent, unique innate immune, lytic, and inflammatory cell death pathway initiated by innate immune sensors and driven by caspases and RIPKs through PANoptosomes. PANoptosomes are multi-protein complexes assembled by innate immune sensor(s) in response to pathogens, PAMPs, DAMPs, cytokines, and homeostatic changes that drive PANoptosis. In this article, we provide methods for molecularly defining distinct cell death pathways, including PANoptosis, using both genetic and chemical approaches through western blot, LDH assay, and microscopy readouts. This procedure allows for the assessment of cell death on the cell population and single-cell levels even without access to genetic models. Having this comprehensive workflow that is more accessible to all labs will improve our ability as a scientific community to accelerate discovery. Using these protocols will help identify new innate immune sensors that drive PANoptosis and define the molecular mechanisms and regulators involved to establish new targets for clinical translation. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Induction and quantification of cell death using live cell imaging</p><p><b>Alternate Protocol 1</b>: Quantification of cell death using LDH</p><p><b>Alternate Protocol 2</b>: Assessment of cell death complexes in single cells using immunofluorescence staining</p><p><b>Basic Protocol 2</b>: Analysis of cell death mechanisms by immunoblots (western blots)</p>\",\"PeriodicalId\":93970,\"journal\":{\"name\":\"Current protocols\",\"volume\":\"4 7\",\"pages\":\"\"},\"PeriodicalIF\":0.0000,\"publicationDate\":\"2024-07-29\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.1112\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Current protocols\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/cpz1.1112\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"\",\"JCRName\":\"\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpz1.1112","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Defining PANoptosis: Biochemical and Mechanistic Evaluation of Innate Immune Cell Death Activation
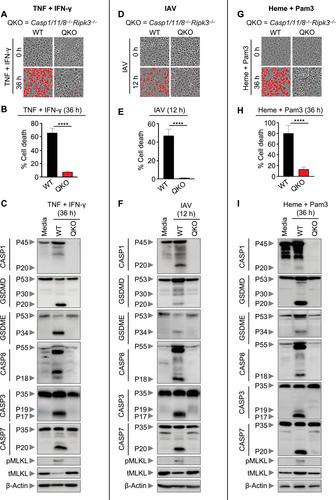
The innate immune system is the first line of host defense. Innate immune activation utilizes pattern recognition receptors to detect pathogens, pathogen-associated and damage-associated molecular patterns (PAMPs and DAMPs), and homeostatic alterations and drives inflammatory signaling pathways and regulated cell death. Cell death activation is critical to eliminate pathogens and aberrant or damaged cells, while excess activation can be linked to inflammation, tissue damage, and disease. Therefore, there is increasing interest in studying cell death mechanisms to understand the underlying biology and identify therapeutic strategies. However, there are significant technical challenges, as many cell death pathways share key molecules with each other, and genetic models where these cell death molecules are deleted remain the gold standard for evaluation. Furthermore, extensive crosstalk has been identified between the cell death pathways pyroptosis, apoptosis, necroptosis, and the more recently characterized PANoptosis, which is defined as a prominent, unique innate immune, lytic, and inflammatory cell death pathway initiated by innate immune sensors and driven by caspases and RIPKs through PANoptosomes. PANoptosomes are multi-protein complexes assembled by innate immune sensor(s) in response to pathogens, PAMPs, DAMPs, cytokines, and homeostatic changes that drive PANoptosis. In this article, we provide methods for molecularly defining distinct cell death pathways, including PANoptosis, using both genetic and chemical approaches through western blot, LDH assay, and microscopy readouts. This procedure allows for the assessment of cell death on the cell population and single-cell levels even without access to genetic models. Having this comprehensive workflow that is more accessible to all labs will improve our ability as a scientific community to accelerate discovery. Using these protocols will help identify new innate immune sensors that drive PANoptosis and define the molecular mechanisms and regulators involved to establish new targets for clinical translation. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1: Induction and quantification of cell death using live cell imaging
Alternate Protocol 1: Quantification of cell death using LDH
Alternate Protocol 2: Assessment of cell death complexes in single cells using immunofluorescence staining
Basic Protocol 2: Analysis of cell death mechanisms by immunoblots (western blots)