下载PDF
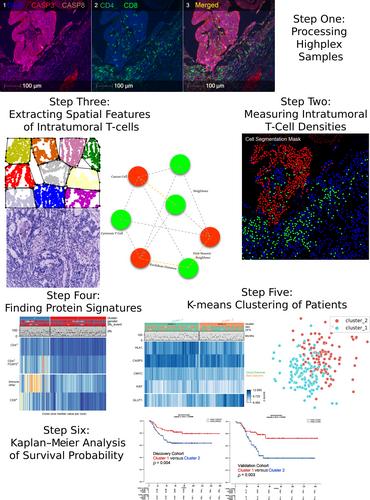
{"title":"浸润 T 细胞对邻近癌细胞的空间影响与 III 期 CRC 患者的预后。","authors":"Mohammadreza Azimi, Sanghee Cho, Emir Bozkurt, Elizabeth McDonough, Batuhan Kisakol, Anna Matveeva, Manuela Salvucci, Heiko Dussmann, Simon McDade, Canan Firat, Nil Urganci, Jinru Shia, Daniel B Longley, Fiona Ginty, Jochen HM Prehn","doi":"10.1002/path.6327","DOIUrl":null,"url":null,"abstract":"<p>Colorectal cancer (CRC) is one of the most frequently occurring cancers, but prognostic biomarkers identifying patients at risk of recurrence are still lacking. In this study, we aimed to investigate in more detail the spatial relationship between intratumoural T cells, cancer cells, and cancer cell hallmarks as prognostic biomarkers in stage III colorectal cancer patients. We conducted multiplexed imaging of 56 protein markers at single-cell resolution on resected fixed tissue from stage III CRC patients who received adjuvant 5-fluorouracil (5FU)-based chemotherapy. Images underwent segmentation for tumour, stroma, and immune cells, and cancer cell ‘state’ protein marker expression was quantified at a cellular level. We developed a Python package for estimation of spatial proximity, nearest neighbour analysis focusing on cancer cell–T-cell interactions at single-cell level. In our discovery cohort (Memorial Sloan Kettering samples), we processed 462 core samples (total number of cells: 1,669,228) from 221 adjuvant 5FU-treated stage III patients. The validation cohort (Huntsville Clearview Cancer Center samples) consisted of 272 samples (total number of cells: 853,398) from 98 stage III CRC patients. While there were trends for an association between the percentage of cytotoxic T cells (across the whole cancer core), it did not reach significance (discovery cohort: <i>p</i> = 0.07; validation cohort: <i>p</i> = 0.19). We next utilised our region-based nearest neighbour approach to determine the spatial relationships between cytotoxic T cells, helper T cells, and cancer cell clusters. In both cohorts, we found that shorter distance between cytotoxic T cells, T helper cells, and cancer cells was significantly associated with increased disease-free survival. An unsupervised trained model that clustered patients based on the median distance between immune cells and cancer cells, as well as protein expression profiles, successfully classified patients into low-risk and high-risk groups (discovery cohort: <i>p</i> = 0.01; validation cohort: <i>p</i> = 0.003). © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 2","pages":"148-159"},"PeriodicalIF":5.6000,"publicationDate":"2024-08-02","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6327","citationCount":"0","resultStr":"{\"title\":\"Spatial effects of infiltrating T cells on neighbouring cancer cells and prognosis in stage III CRC patients\",\"authors\":\"Mohammadreza Azimi, Sanghee Cho, Emir Bozkurt, Elizabeth McDonough, Batuhan Kisakol, Anna Matveeva, Manuela Salvucci, Heiko Dussmann, Simon McDade, Canan Firat, Nil Urganci, Jinru Shia, Daniel B Longley, Fiona Ginty, Jochen HM Prehn\",\"doi\":\"10.1002/path.6327\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Colorectal cancer (CRC) is one of the most frequently occurring cancers, but prognostic biomarkers identifying patients at risk of recurrence are still lacking. In this study, we aimed to investigate in more detail the spatial relationship between intratumoural T cells, cancer cells, and cancer cell hallmarks as prognostic biomarkers in stage III colorectal cancer patients. We conducted multiplexed imaging of 56 protein markers at single-cell resolution on resected fixed tissue from stage III CRC patients who received adjuvant 5-fluorouracil (5FU)-based chemotherapy. Images underwent segmentation for tumour, stroma, and immune cells, and cancer cell ‘state’ protein marker expression was quantified at a cellular level. We developed a Python package for estimation of spatial proximity, nearest neighbour analysis focusing on cancer cell–T-cell interactions at single-cell level. In our discovery cohort (Memorial Sloan Kettering samples), we processed 462 core samples (total number of cells: 1,669,228) from 221 adjuvant 5FU-treated stage III patients. The validation cohort (Huntsville Clearview Cancer Center samples) consisted of 272 samples (total number of cells: 853,398) from 98 stage III CRC patients. While there were trends for an association between the percentage of cytotoxic T cells (across the whole cancer core), it did not reach significance (discovery cohort: <i>p</i> = 0.07; validation cohort: <i>p</i> = 0.19). We next utilised our region-based nearest neighbour approach to determine the spatial relationships between cytotoxic T cells, helper T cells, and cancer cell clusters. In both cohorts, we found that shorter distance between cytotoxic T cells, T helper cells, and cancer cells was significantly associated with increased disease-free survival. An unsupervised trained model that clustered patients based on the median distance between immune cells and cancer cells, as well as protein expression profiles, successfully classified patients into low-risk and high-risk groups (discovery cohort: <i>p</i> = 0.01; validation cohort: <i>p</i> = 0.003). © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"264 2\",\"pages\":\"148-159\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2024-08-02\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6327\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6327\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6327","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用