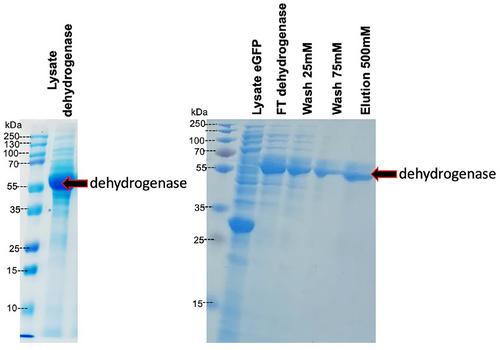
{"title":"超快 His 标记蛋白质纯化","authors":"Xuan Luo, Arjun S. Pamidi, Zoe Gardner, Fayed Abdullah Alrashaidi, Colin L. Raston, Gregory A. Weiss","doi":"10.1002/cpz1.70006","DOIUrl":null,"url":null,"abstract":"<p>This article details how to use a vortex fluidic device (VFD) to accelerate protein purification via immobilized metal affinity chromatography (IMAC). Building upon a previous report of VFD-based purification, we introduce a membrane insert to simplify the purification protocol and the resin recovery step. This new platform can be adapted to different types of IMAC resins and purification membranes. Proteins can be purified directly from clarified lysate, non-clarified lysate, and even non-lysed cultures without concerns of system clogging. Strong binding between the Ni<sup>2+</sup> and the target protein's His<sub>6</sub>-tag effectively captures the target protein on IMAC resins or membranes placed in the VFD. Continuous flow of different solutions through the VFD allows dynamic binding, washing, and elution of the target protein. Furthermore, the system dramatically accelerates protein purification; a typical purification from cell lysate requires approximately 4 min. Herein, we demonstrate the single-step purification of two His<sub>6</sub>-tagged proteins from both clarified and non-clarified cell lysates without requiring batch binding. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Preparation of the resin-loaded membrane insert and the vortex fluidic device (VFD) setup prior to purification</p><p><b>Basic Protocol 2</b>: Purification of His<sub>6</sub>-tagged proteins using the VFD</p><p><b>Alternate Protocol</b>: VFD-mediated His<sub>6</sub>-tagged protein purification from non-clarified lysate</p><p><b>Support Protocol</b>: Preparation of chemically modified glass fiber membrane for VFD-mediated immobilized metal affinity chromatography purification</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"4 9","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2024-09-20","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.70006","citationCount":"0","resultStr":"{\"title\":\"Ultrafast His-Tagged Protein Purification\",\"authors\":\"Xuan Luo, Arjun S. Pamidi, Zoe Gardner, Fayed Abdullah Alrashaidi, Colin L. Raston, Gregory A. Weiss\",\"doi\":\"10.1002/cpz1.70006\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>This article details how to use a vortex fluidic device (VFD) to accelerate protein purification via immobilized metal affinity chromatography (IMAC). Building upon a previous report of VFD-based purification, we introduce a membrane insert to simplify the purification protocol and the resin recovery step. This new platform can be adapted to different types of IMAC resins and purification membranes. Proteins can be purified directly from clarified lysate, non-clarified lysate, and even non-lysed cultures without concerns of system clogging. Strong binding between the Ni<sup>2+</sup> and the target protein's His<sub>6</sub>-tag effectively captures the target protein on IMAC resins or membranes placed in the VFD. Continuous flow of different solutions through the VFD allows dynamic binding, washing, and elution of the target protein. Furthermore, the system dramatically accelerates protein purification; a typical purification from cell lysate requires approximately 4 min. Herein, we demonstrate the single-step purification of two His<sub>6</sub>-tagged proteins from both clarified and non-clarified cell lysates without requiring batch binding. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Preparation of the resin-loaded membrane insert and the vortex fluidic device (VFD) setup prior to purification</p><p><b>Basic Protocol 2</b>: Purification of His<sub>6</sub>-tagged proteins using the VFD</p><p><b>Alternate Protocol</b>: VFD-mediated His<sub>6</sub>-tagged protein purification from non-clarified lysate</p><p><b>Support Protocol</b>: Preparation of chemically modified glass fiber membrane for VFD-mediated immobilized metal affinity chromatography purification</p>\",\"PeriodicalId\":93970,\"journal\":{\"name\":\"Current protocols\",\"volume\":\"4 9\",\"pages\":\"\"},\"PeriodicalIF\":0.0000,\"publicationDate\":\"2024-09-20\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.70006\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Current protocols\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/cpz1.70006\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"\",\"JCRName\":\"\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpz1.70006","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0