下载PDF
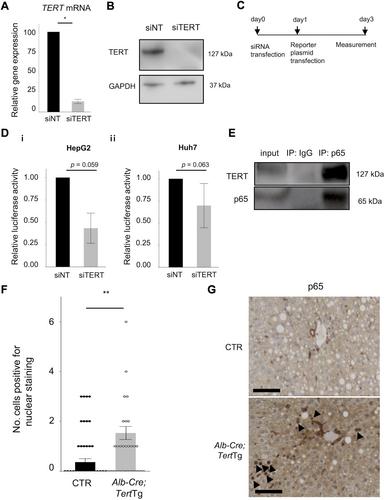
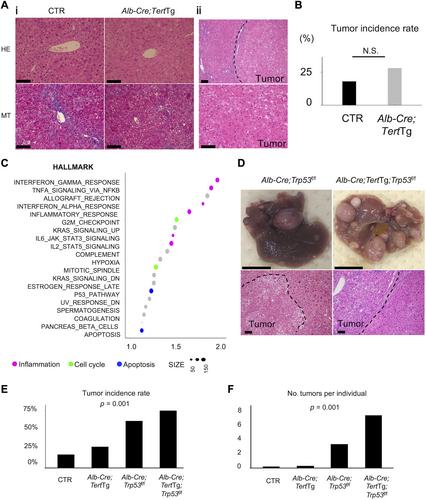
{"title":"TERT 上调会通过降解 p21 促进细胞增殖,并增加致癌可能性。","authors":"Masako Mishima, Atsushi Takai, Haruhiko Takeda, Eriko Iguchi, Shigeharu Nakano, Yosuke Fujii, Masayuki Ueno, Takahiko Ito, Mari Teramura, Yuji Eso, Takahiro Shimizu, Takahisa Maruno, Shizu Hidema, Katsuhiko Nishimori, Hiroyuki Marusawa, Etsuro Hatano, Hiroshi Seno","doi":"10.1002/path.6351","DOIUrl":null,"url":null,"abstract":"<p>Telomerase reverse transcriptase (<i>TERT</i>) gene aberration is detectable in >80% of cases with hepatocellular carcinoma (HCC). TERT reactivation is essential for cellular immortalization because it stabilizes telomere length, although the role of TERT in hepatocarcinogenesis remains unelucidated. To elucidate the significance of aberrant TERT expression in hepatocytes in inflammation-associated hepatocarcinogenesis, we generated <i>Alb-Cre;Tert</i>Tg mice, which overexpress TERT in the liver and examined their phenotype during chronic inflammation. Based on transcriptome data from the liver tissue of <i>Alb-Cre;Tert</i>Tg mice, we examined the role of TERT in hepatocarcinogenesis <i>in vitro</i>. We also evaluated the relationship between TERT and cell-cycle-related molecules, including p21, in HCC samples. The liver tumor development rate was increased by TERT overexpression during chronic inflammation, especially in the absence of p53 function. Gene set enrichment analysis of liver tissues revealed that gene sets related to TNF-NFκB signaling, cell cycle, and apoptosis were upregulated in <i>Alb-Cre;Tert</i>Tg liver. A luciferase reporter assay and immunoprecipitation revealed that TERT interacted with NFκB p65 and enhanced NFκB promoter activity. On the other hand, TERT formed protein complexes with p21, cyclin A2, and cyclin E and promoted ubiquitin-mediated degradation of p21, specifically in the G1 phase. In the clinical HCC samples, TERT was highly expressed but p21 was conversely downregulated, and TERT expression was associated with the upregulation of molecules related to the cell cycle. Taken together, the aberrant upregulation of TERT increased NFκB promoter activity and promoted cell cycle progression via p21 ubiquitination, leading to hepatocarcinogenesis. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 3","pages":"318-331"},"PeriodicalIF":5.6000,"publicationDate":"2024-09-27","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6351","citationCount":"0","resultStr":"{\"title\":\"TERT upregulation promotes cell proliferation via degradation of p21 and increases carcinogenic potential\",\"authors\":\"Masako Mishima, Atsushi Takai, Haruhiko Takeda, Eriko Iguchi, Shigeharu Nakano, Yosuke Fujii, Masayuki Ueno, Takahiko Ito, Mari Teramura, Yuji Eso, Takahiro Shimizu, Takahisa Maruno, Shizu Hidema, Katsuhiko Nishimori, Hiroyuki Marusawa, Etsuro Hatano, Hiroshi Seno\",\"doi\":\"10.1002/path.6351\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Telomerase reverse transcriptase (<i>TERT</i>) gene aberration is detectable in >80% of cases with hepatocellular carcinoma (HCC). TERT reactivation is essential for cellular immortalization because it stabilizes telomere length, although the role of TERT in hepatocarcinogenesis remains unelucidated. To elucidate the significance of aberrant TERT expression in hepatocytes in inflammation-associated hepatocarcinogenesis, we generated <i>Alb-Cre;Tert</i>Tg mice, which overexpress TERT in the liver and examined their phenotype during chronic inflammation. Based on transcriptome data from the liver tissue of <i>Alb-Cre;Tert</i>Tg mice, we examined the role of TERT in hepatocarcinogenesis <i>in vitro</i>. We also evaluated the relationship between TERT and cell-cycle-related molecules, including p21, in HCC samples. The liver tumor development rate was increased by TERT overexpression during chronic inflammation, especially in the absence of p53 function. Gene set enrichment analysis of liver tissues revealed that gene sets related to TNF-NFκB signaling, cell cycle, and apoptosis were upregulated in <i>Alb-Cre;Tert</i>Tg liver. A luciferase reporter assay and immunoprecipitation revealed that TERT interacted with NFκB p65 and enhanced NFκB promoter activity. On the other hand, TERT formed protein complexes with p21, cyclin A2, and cyclin E and promoted ubiquitin-mediated degradation of p21, specifically in the G1 phase. In the clinical HCC samples, TERT was highly expressed but p21 was conversely downregulated, and TERT expression was associated with the upregulation of molecules related to the cell cycle. Taken together, the aberrant upregulation of TERT increased NFκB promoter activity and promoted cell cycle progression via p21 ubiquitination, leading to hepatocarcinogenesis. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"264 3\",\"pages\":\"318-331\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2024-09-27\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6351\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6351\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6351","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用