下载PDF
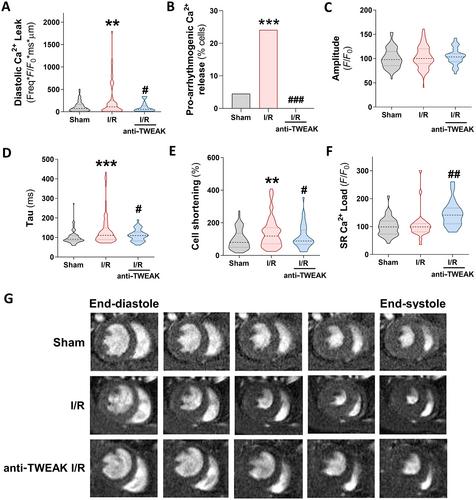
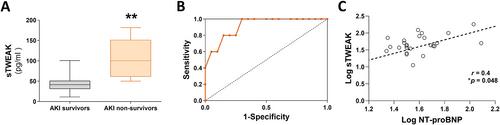
{"title":"靶向TWEAK-Fn14通路可预防急性肾损伤后心脏钙处理功能障碍","authors":"Jonay Poveda, Laura González-Lafuente, Sara Vázquez-Sánchez, Elisa Mercado-García, Elena Rodríguez-Sánchez, Inés García-Consuegra, Ana Belén Sanz, Julián Segura, María Fernández-Velasco, Fernando Liaño, Luis M Ruilope, Gema Ruiz-Hurtado","doi":"10.1002/path.6200","DOIUrl":null,"url":null,"abstract":"<p>Heart and kidney have a closely interrelated pathophysiology. Acute kidney injury (AKI) is associated with significantly increased rates of cardiovascular events, a relationship defined as cardiorenal syndrome type 3 (CRS3). The underlying mechanisms that trigger heart disease remain, however, unknown, particularly concerning the clinical impact of AKI on cardiac outcomes and overall mortality. Tumour necrosis factor-like weak inducer of apoptosis (TWEAK) and its receptor fibroblast growth factor-inducible 14 (Fn14) are independently involved in the pathogenesis of both heart and kidney failure, and recent studies have proposed TWEAK as a possible therapeutic target; however, its specific role in cardiac damage associated with CRS3 remains to be clarified. Firstly, we demonstrated in a retrospective longitudinal clinical study that soluble TWEAK plasma levels were a predictive biomarker of mortality in patients with AKI. Furthermore, the exogenous application of TWEAK to native ventricular cardiomyocytes induced relevant calcium (Ca<sup>2+</sup>) handling alterations. Next, we investigated the role of the TWEAK–Fn14 axis in cardiomyocyte function following renal ischaemia–reperfusion (I/R) injury in mice. We observed that TWEAK–Fn14 signalling was activated in the hearts of AKI mice. Mice also showed significantly altered intra-cardiomyocyte Ca<sup>2+</sup> handling and arrhythmogenic Ca<sup>2+</sup> events through an impairment in sarcoplasmic reticulum Ca<sup>2+</sup>-adenosine triphosphatase 2a pump (SERCA<sub>2a</sub>) and ryanodine receptor (RyR<sub>2</sub>) function. Administration of anti-TWEAK antibody after reperfusion significantly improved alterations in Ca<sup>2+</sup> cycling and arrhythmogenic events and prevented SERCA<sub>2a</sub> and RyR<sub>2</sub> modifications. In conclusion, this study establishes the relevance of the TWEAK–Fn14 pathway in cardiac dysfunction linked to CRS3, both as a predictor of mortality in patients with AKI and as a Ca<sup>2+</sup> mishandling inducer in cardiomyocytes, and highlights the cardioprotective benefits of TWEAK targeting in CRS3. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"261 4","pages":"427-441"},"PeriodicalIF":5.6000,"publicationDate":"2023-09-30","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://pathsocjournals.onlinelibrary.wiley.com/doi/epdf/10.1002/path.6200","citationCount":"0","resultStr":"{\"title\":\"Targeting the TWEAK–Fn14 pathway prevents dysfunction in cardiac calcium handling after acute kidney injury\",\"authors\":\"Jonay Poveda, Laura González-Lafuente, Sara Vázquez-Sánchez, Elisa Mercado-García, Elena Rodríguez-Sánchez, Inés García-Consuegra, Ana Belén Sanz, Julián Segura, María Fernández-Velasco, Fernando Liaño, Luis M Ruilope, Gema Ruiz-Hurtado\",\"doi\":\"10.1002/path.6200\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Heart and kidney have a closely interrelated pathophysiology. Acute kidney injury (AKI) is associated with significantly increased rates of cardiovascular events, a relationship defined as cardiorenal syndrome type 3 (CRS3). The underlying mechanisms that trigger heart disease remain, however, unknown, particularly concerning the clinical impact of AKI on cardiac outcomes and overall mortality. Tumour necrosis factor-like weak inducer of apoptosis (TWEAK) and its receptor fibroblast growth factor-inducible 14 (Fn14) are independently involved in the pathogenesis of both heart and kidney failure, and recent studies have proposed TWEAK as a possible therapeutic target; however, its specific role in cardiac damage associated with CRS3 remains to be clarified. Firstly, we demonstrated in a retrospective longitudinal clinical study that soluble TWEAK plasma levels were a predictive biomarker of mortality in patients with AKI. Furthermore, the exogenous application of TWEAK to native ventricular cardiomyocytes induced relevant calcium (Ca<sup>2+</sup>) handling alterations. Next, we investigated the role of the TWEAK–Fn14 axis in cardiomyocyte function following renal ischaemia–reperfusion (I/R) injury in mice. We observed that TWEAK–Fn14 signalling was activated in the hearts of AKI mice. Mice also showed significantly altered intra-cardiomyocyte Ca<sup>2+</sup> handling and arrhythmogenic Ca<sup>2+</sup> events through an impairment in sarcoplasmic reticulum Ca<sup>2+</sup>-adenosine triphosphatase 2a pump (SERCA<sub>2a</sub>) and ryanodine receptor (RyR<sub>2</sub>) function. Administration of anti-TWEAK antibody after reperfusion significantly improved alterations in Ca<sup>2+</sup> cycling and arrhythmogenic events and prevented SERCA<sub>2a</sub> and RyR<sub>2</sub> modifications. In conclusion, this study establishes the relevance of the TWEAK–Fn14 pathway in cardiac dysfunction linked to CRS3, both as a predictor of mortality in patients with AKI and as a Ca<sup>2+</sup> mishandling inducer in cardiomyocytes, and highlights the cardioprotective benefits of TWEAK targeting in CRS3. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"261 4\",\"pages\":\"427-441\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2023-09-30\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://pathsocjournals.onlinelibrary.wiley.com/doi/epdf/10.1002/path.6200\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6200\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6200","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用