透明质酸介导的运动受体(RHAMM)定义了与肿瘤进展相关的侵袭性生态位,并预测乳腺癌患者的不良预后
Sarah E Tarullo, Yuyu He, Claire Daughters, Todd P Knutson, Christine M Henzler, Matthew A Price, Ryan Shanley, Patrice Witschen, Cornelia Tolg, Rachael E Kaspar, Caroline Hallstrom, Lyubov Gittsovich, Megan L Sulciner, Xihong Zhang, Colleen L Forster, Carol A Lange, Oleg Shats, Michelle Desler, Kenneth H Cowan, Douglas Yee, Kathryn L Schwertfeger, Eva A Turley, James B McCarthy, Andrew C Nelson
下载PDF
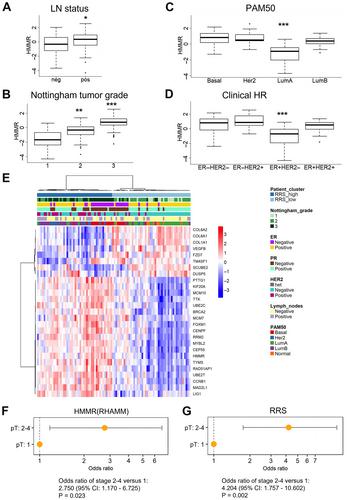
{"title":"透明质酸介导的运动受体(RHAMM)定义了与肿瘤进展相关的侵袭性生态位,并预测乳腺癌患者的不良预后","authors":"Sarah E Tarullo, Yuyu He, Claire Daughters, Todd P Knutson, Christine M Henzler, Matthew A Price, Ryan Shanley, Patrice Witschen, Cornelia Tolg, Rachael E Kaspar, Caroline Hallstrom, Lyubov Gittsovich, Megan L Sulciner, Xihong Zhang, Colleen L Forster, Carol A Lange, Oleg Shats, Michelle Desler, Kenneth H Cowan, Douglas Yee, Kathryn L Schwertfeger, Eva A Turley, James B McCarthy, Andrew C Nelson","doi":"10.1002/path.6082","DOIUrl":null,"url":null,"abstract":"<p>Breast cancer invasion and metastasis result from a complex interplay between tumor cells and the tumor microenvironment (TME). Key oncogenic changes in the TME include aberrant synthesis, processing, and signaling of hyaluronan (HA). Hyaluronan-mediated motility receptor (RHAMM, CD168; <i>HMMR</i>) is an HA receptor enabling tumor cells to sense and respond to this aberrant TME during breast cancer progression. Previous studies have associated RHAMM expression with breast tumor progression; however, cause and effect mechanisms are incompletely established. Focused gene expression analysis of an internal breast cancer patient cohort confirmed that increased <i>RHAMM</i> expression correlates with aggressive clinicopathological features. To probe mechanisms, we developed a novel 27-gene RHAMM-related signature (RRS) by intersecting differentially expressed genes in lymph node (LN)-positive patient cases with the transcriptome of a RHAMM-dependent model of cell transformation, which we validated in an independent cohort. We demonstrate that the RRS predicts for poor survival and is enriched for cell cycle and TME-interaction pathways. Further analyses using CRISPR/Cas9-generated <i>RHAMM</i><sup>−/−</sup> breast cancer cells provided direct evidence that RHAMM promotes invasion <i>in vitro</i> and <i>in vivo</i>. Immunohistochemistry studies highlighted heterogeneous RHAMM protein expression, and spatial transcriptomics associated the RRS with RHAMM-high microanatomic foci. We conclude that RHAMM upregulation leads to the formation of ‘invasive niches’, which are enriched in RRS-related pathways that drive invasion and could be targeted to limit invasive progression and improve patient outcomes. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"260 3","pages":"289-303"},"PeriodicalIF":5.2000,"publicationDate":"2023-04-26","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://pathsocjournals.onlinelibrary.wiley.com/doi/epdf/10.1002/path.6082","citationCount":"1","resultStr":"{\"title\":\"Receptor for hyaluronan-mediated motility (RHAMM) defines an invasive niche associated with tumor progression and predicts poor outcomes in breast cancer patients\",\"authors\":\"Sarah E Tarullo, Yuyu He, Claire Daughters, Todd P Knutson, Christine M Henzler, Matthew A Price, Ryan Shanley, Patrice Witschen, Cornelia Tolg, Rachael E Kaspar, Caroline Hallstrom, Lyubov Gittsovich, Megan L Sulciner, Xihong Zhang, Colleen L Forster, Carol A Lange, Oleg Shats, Michelle Desler, Kenneth H Cowan, Douglas Yee, Kathryn L Schwertfeger, Eva A Turley, James B McCarthy, Andrew C Nelson\",\"doi\":\"10.1002/path.6082\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Breast cancer invasion and metastasis result from a complex interplay between tumor cells and the tumor microenvironment (TME). Key oncogenic changes in the TME include aberrant synthesis, processing, and signaling of hyaluronan (HA). Hyaluronan-mediated motility receptor (RHAMM, CD168; <i>HMMR</i>) is an HA receptor enabling tumor cells to sense and respond to this aberrant TME during breast cancer progression. Previous studies have associated RHAMM expression with breast tumor progression; however, cause and effect mechanisms are incompletely established. Focused gene expression analysis of an internal breast cancer patient cohort confirmed that increased <i>RHAMM</i> expression correlates with aggressive clinicopathological features. To probe mechanisms, we developed a novel 27-gene RHAMM-related signature (RRS) by intersecting differentially expressed genes in lymph node (LN)-positive patient cases with the transcriptome of a RHAMM-dependent model of cell transformation, which we validated in an independent cohort. We demonstrate that the RRS predicts for poor survival and is enriched for cell cycle and TME-interaction pathways. Further analyses using CRISPR/Cas9-generated <i>RHAMM</i><sup>−/−</sup> breast cancer cells provided direct evidence that RHAMM promotes invasion <i>in vitro</i> and <i>in vivo</i>. Immunohistochemistry studies highlighted heterogeneous RHAMM protein expression, and spatial transcriptomics associated the RRS with RHAMM-high microanatomic foci. We conclude that RHAMM upregulation leads to the formation of ‘invasive niches’, which are enriched in RRS-related pathways that drive invasion and could be targeted to limit invasive progression and improve patient outcomes. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"260 3\",\"pages\":\"289-303\"},\"PeriodicalIF\":5.2000,\"publicationDate\":\"2023-04-26\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://pathsocjournals.onlinelibrary.wiley.com/doi/epdf/10.1002/path.6082\",\"citationCount\":\"1\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6082\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6082","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 1
引用
批量引用