下载PDF
{"title":"肠道白色念珠菌过度生长加剧博莱霉素诱导的肺纤维化失调小鼠","authors":"Takahiro Yamada, Taku Nakashima, Takeshi Masuda, Shinjiro Sakamoto, Kakuhiro Yamaguchi, Yasushi Horimasu, Shintaro Miyamoto, Hiroshi Iwamoto, Kazunori Fujitaka, Hironobu Hamada, Nobuhiko Kamada, Noboru Hattori","doi":"10.1002/path.6169","DOIUrl":null,"url":null,"abstract":"Increasing evidence indicates an interaction between the intestinal microbiota and diseases in distal organs. However, the relationship between pulmonary fibrosis and the intestinal microbiota, especially intestinal fungal microbiota, is poorly understood. Thus, this study aimed to determine the effects of changes in the intestinal fungal microbiota on the pathogenesis of pulmonary fibrosis. Mice with intestinal overgrowth of Candida albicans, which was established by oral administration of antibiotics plus C. albicans, showed accelerated bleomycin‐induced pulmonary fibrosis relative to the control mice (i.e. without C. albicans treatment). In addition, the mice with intestinal overgrowth of C. albicans showed enhanced Th17‐type immunity, and treatment with IL‐17A‐neutralizing antibody alleviated pulmonary fibrosis in these mice but not in the control mice. This result indicates that IL‐17A is involved in the pathogenesis of C. albicans‐exacerbated pulmonary fibrosis. Even before bleomycin treatment, the expression of Rorc, the master regulator of Th17, was already upregulated in the pulmonary lymphocytes of the mice with intestinal overgrowth of C. albicans. Subsequent administration of bleomycin triggered these Th17‐skewed lymphocytes to produce IL‐17A, which enhanced endothelial–mesenchymal transition. These results suggest that intestinal overgrowth of C. albicans exacerbates pulmonary fibrosis via IL‐17A‐mediated endothelial–mesenchymal transition. Thus, it might be a potential therapeutic target in pulmonary fibrosis. This study may serve as a basis for using intestinal fungal microbiota as novel therapeutic targets in pulmonary fibrosis. © 2023 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"261 2","pages":"227-237"},"PeriodicalIF":5.6000,"publicationDate":"2023-08-11","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6169","citationCount":"0","resultStr":"{\"title\":\"Intestinal overgrowth of Candida albicans exacerbates bleomycin-induced pulmonary fibrosis in mice with dysbiosis\",\"authors\":\"Takahiro Yamada, Taku Nakashima, Takeshi Masuda, Shinjiro Sakamoto, Kakuhiro Yamaguchi, Yasushi Horimasu, Shintaro Miyamoto, Hiroshi Iwamoto, Kazunori Fujitaka, Hironobu Hamada, Nobuhiko Kamada, Noboru Hattori\",\"doi\":\"10.1002/path.6169\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"Increasing evidence indicates an interaction between the intestinal microbiota and diseases in distal organs. However, the relationship between pulmonary fibrosis and the intestinal microbiota, especially intestinal fungal microbiota, is poorly understood. Thus, this study aimed to determine the effects of changes in the intestinal fungal microbiota on the pathogenesis of pulmonary fibrosis. Mice with intestinal overgrowth of Candida albicans, which was established by oral administration of antibiotics plus C. albicans, showed accelerated bleomycin‐induced pulmonary fibrosis relative to the control mice (i.e. without C. albicans treatment). In addition, the mice with intestinal overgrowth of C. albicans showed enhanced Th17‐type immunity, and treatment with IL‐17A‐neutralizing antibody alleviated pulmonary fibrosis in these mice but not in the control mice. This result indicates that IL‐17A is involved in the pathogenesis of C. albicans‐exacerbated pulmonary fibrosis. Even before bleomycin treatment, the expression of Rorc, the master regulator of Th17, was already upregulated in the pulmonary lymphocytes of the mice with intestinal overgrowth of C. albicans. Subsequent administration of bleomycin triggered these Th17‐skewed lymphocytes to produce IL‐17A, which enhanced endothelial–mesenchymal transition. These results suggest that intestinal overgrowth of C. albicans exacerbates pulmonary fibrosis via IL‐17A‐mediated endothelial–mesenchymal transition. Thus, it might be a potential therapeutic target in pulmonary fibrosis. This study may serve as a basis for using intestinal fungal microbiota as novel therapeutic targets in pulmonary fibrosis. © 2023 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"261 2\",\"pages\":\"227-237\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2023-08-11\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6169\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6169\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6169","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
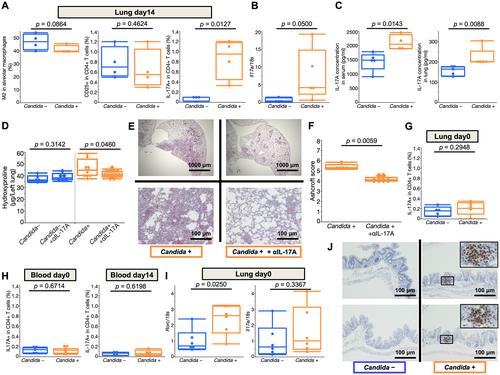
Intestinal overgrowth of Candida albicans exacerbates bleomycin-induced pulmonary fibrosis in mice with dysbiosis
Increasing evidence indicates an interaction between the intestinal microbiota and diseases in distal organs. However, the relationship between pulmonary fibrosis and the intestinal microbiota, especially intestinal fungal microbiota, is poorly understood. Thus, this study aimed to determine the effects of changes in the intestinal fungal microbiota on the pathogenesis of pulmonary fibrosis. Mice with intestinal overgrowth of Candida albicans, which was established by oral administration of antibiotics plus C. albicans, showed accelerated bleomycin‐induced pulmonary fibrosis relative to the control mice (i.e. without C. albicans treatment). In addition, the mice with intestinal overgrowth of C. albicans showed enhanced Th17‐type immunity, and treatment with IL‐17A‐neutralizing antibody alleviated pulmonary fibrosis in these mice but not in the control mice. This result indicates that IL‐17A is involved in the pathogenesis of C. albicans‐exacerbated pulmonary fibrosis. Even before bleomycin treatment, the expression of Rorc, the master regulator of Th17, was already upregulated in the pulmonary lymphocytes of the mice with intestinal overgrowth of C. albicans. Subsequent administration of bleomycin triggered these Th17‐skewed lymphocytes to produce IL‐17A, which enhanced endothelial–mesenchymal transition. These results suggest that intestinal overgrowth of C. albicans exacerbates pulmonary fibrosis via IL‐17A‐mediated endothelial–mesenchymal transition. Thus, it might be a potential therapeutic target in pulmonary fibrosis. This study may serve as a basis for using intestinal fungal microbiota as novel therapeutic targets in pulmonary fibrosis. © 2023 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.