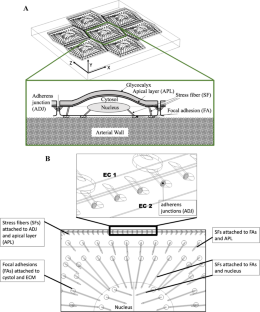
Disturbed flow promotes progression of atherosclerosis at particular regions of arteries where the recent studies show the arterial wall becomes stiffer. Objective of this study is to show how mechanotransduction in subcellular organelles of endothelial cells (ECs) will alter with changes in blood flow profiles applied on ECs surface and mechanical properties of arterial wall where ECs are attached to. We will examine the exposure of ECs to atherogenic flow profiles (disturbed flow) and non-atherogenic flow profiles (purely forward flow), while stiffness and viscoelasticity of arterial wall will change. A multicomponent model of endothelial cell monolayer was applied to quantify the response of subcellular organelles to the changes in their microenvironment. Our results show that arterial stiffening alters mechanotransduction in intra/inter-cellular organelles of ECs by slight increase in the transmitted stresses, particularly over central stress fibers (SFs). We also observed that degradation of glycocalyx and exposure to non-atherogenic flow profiles result in significantly higher stresses in subcellular organelles, while degradation of glycocalyx and exposure to atherogenic flow profiles result in dramatically lower stresses in the organelles. Moreover, we show that increasing the arterial wall viscoelasticity leads to slight increase in the stresses transmitted to subcellular organelles. FAs are particularly influenced with the changes in the arterial wall properties and viscoelasticity. Our study suggests that changes in viscoelasticity of arterial wall and degradation state of glycocalyx have to be considered along with arterial stiffening in designing more efficient treatment strategies for atherosclerosis. Our study provides insight into significant role of mechanotransduction in the localization of atherosclerosis by quantifying the role of ECs mechanosensors and suggests that mechanotransduction may play a key role in design of more efficient and precision therapeutics to slow down or block the progression of atherosclerosis.