The functional deterioration and loss of motor neurons are tightly associated with degenerative motor neuron diseases and aging-related muscle wasting. Motor neuron diseases or aging-related muscle wasting in turn contribute to increased risk of adverse health outcomes in the elderly. Cdon (cell adhesion molecule-downregulated oncogene) belongs to the immunoglobulin superfamily of cell adhesion molecule and plays essential roles in multiple signalling pathways, including sonic hedgehog (Shh), netrin, and cadherin-mediated signalling. Cdon as a Shh coreceptor plays a critical role in motor neuron specification during embryonic development. However, its role in adult motor neuron function is unknown.
Hb9-Cre recombinase-driven motor neuron-specific Cdon deficient mice (mnKO) and a compound mutant mice (mnKO::SOD1G93A) were generated to investigate the role of Cdon in motor neuron degeneration. Motor neuron regeneration was examined by using a sciatic nerve crush injury model. To investigate the phenotype, physical activity, compound muscle action potential, immunostaining, and transmission electron microscopy were carried out. In the mechanism study, RNA sequencing and RNA/protein analyses were employed.
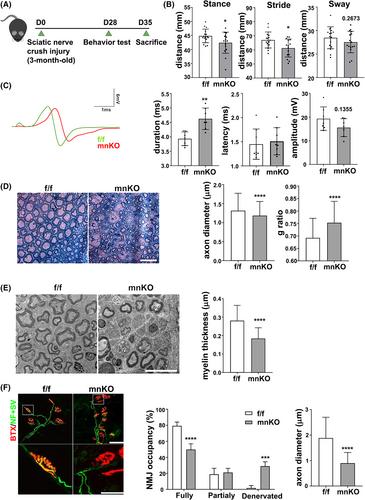
Mice lacking Cdon in motor neurons exhibited middle age onset lethality and aging-related decline in motor function. In the sciatic nerve crush injury model, mnKO mice exhibited an impairment in motor function recovery evident by prolonged compound muscle action potential duration (4.63 ± 0.35 vs. 3.93 ± 0.22 s for f/f, P < 0.01) and physical activity. Consistently, neuromuscular junctions of mnKO muscles were incompletely occupied (49.79 ± 5.74 vs. 79.39 ± 3.77% fully occupied neuromuscular junctions for f/f, P < 0.0001), suggesting an impaired reinnervation. The transmission electron microscopy analysis revealed that mnKO sciatic nerves had smaller axon diameter (0.88 ± 0.13 vs. 1.43 ± 0.48 μm for f/f, P < 0.0001) and myelination defects. RNA sequencing of mnKO lumbar spinal cords showed alteration in genes related to neurogenesis, inflammation and cell death. Among the altered genes, ErbB4 and FgfR expressions were significantly altered in mnKO as well as in Cdon-depleted NSC34 motor neuron cells. Consistently, Cdon-depleted NSC34 cells exhibited elevated levels of cleaved Caspase3 and γH2AX proteins, as well as Bax transcription. Cdon-depleted NSC34 cells also exhibited impaired activation of Akt in response to neuregulin-1 (NRG1) treatment.
Our current data demonstrate the functional importance of Cdon in motor neuron function and nerve repair. Cdon ablation causes alterations in neurotrophin signalling that leads to motor neuron degeneration.