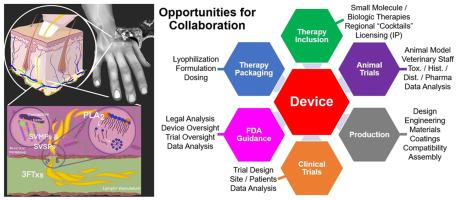
The timely administration of antivenom is the most effective method currently available to reduce the burden of snakebite envenoming (SBE), a neglected tropical disease that most often affects rural agricultural global populations. There is increasing interest in the development of adjunctive small molecule and biologic therapeutics that target the most problematic venom components to bridge the time-gap between initial SBE and the administration of antivenom. Unique combinations of these therapeutics could provide relief from the toxic effects of regional groupings of medically relevant snake species. The application a PRISMA/PICO literature search methodology demonstrated an increasing interest in the rapid administration of therapies to improve patient symptoms and outcomes after SBE. Advice from expert interviews and considerations regarding the potential routes of therapy administration, anatomical bite location, and species-specific venom delivery have provided a framework to identify ideal metrics and potential hurdles for the development of a field-based medical device that could be used immediately after SBE to deliver adjunctive therapies. The use of subcutaneous (SC) or intramuscular (IM) injection were identified as potential routes of administration of both small molecule and biologic therapies. The development of a field-based medical device for the delivery of adjunctive SBE therapies presents unique challenges that will require a collaborative and transdisciplinary approach to be successful.