Anaphylaxis is a sudden multisystem allergic reaction which may result in a fatal outcome if not treated promptly. Guidelines worldwide suggest intramuscular adrenaline as the first-line treatment for anaphylaxis outside a perioperative reaction. Adrenaline autoinjectors (AAIs) are widely used self-administrable devices, especially in community settings. Different commercial AAIs have been authorized to be marketed in Europe. For an AAI to be efficacious, a rapid adrenaline delivery in patients, including those who are overweight or obese, resulting in an optimal cardiovascular (CV) response, is a key feature. AAIs are designed to achieve this requirement, which is reflected in their differing functional properties such as primary container selection, drug delivery mechanism (cartridge-or syringe-based), needle length, needle gauge, and adrenaline dose (150 μg, 300 μg, or 500 μg). However, the differences in functional properties across these devices may play a critical role in achieving these requirements as well as the differences in ergonomics in the handling of these devices.
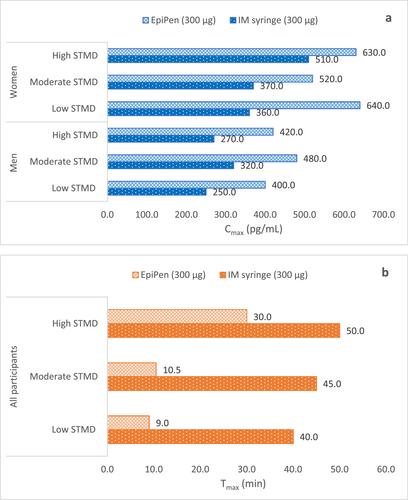
Considering the dynamic pharmacokinetic/pharmacodynamic (PK/PD) profiles of different AAIs marketed in Europe and their effect on adrenaline delivery, the expert panel, also serving as author for this paper have carried out a detailed analysis of the PK/PD profiles of four AAIs, namely, Anapen, Emerade, EpiPen, and Jext, to delineate the adrenaline delivery and their subsequent physiological effects on the backdrop of device characteristics, dose strength, and the skin-to-muscle distances of the participants.