Microcirculatory perfusion disorder and inflammatory response are critical links in acute kidney injury (AKI). We aim to construct anti-vascular cell adhesion molecule-1(VCAM-1) targeted microbubbles (TM) to monitor renal microcirculatory perfusion and inflammatory response.
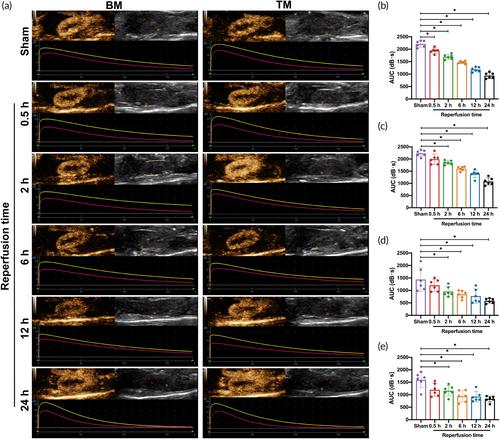
TM carrying VCAM-1 polypeptide was constructed by biological coupling. The binding ability of TM to human umbilical vein endothelial cells (HUVECs) was detected. Bilateral renal ischemia–reperfusion injury (IRI) models of mice were established to evaluate microcirculatory perfusion and inflammatory response using TM. Thirty-six mice were randomly divided into six groups according to the different reperfusion time (0.5, 2, 6, 12, and 24 h) and sham-operated group (Sham group). The correlation of TM imaging with serum and histopathological biomarkers was investigated.
TM has advantages such as uniform distribution, regular shape, high stability, and good biosafety. TM could bind specifically to VCAM-1 molecule expressed by tumor necrosis factor-alpha (TNF-α)-treated HUVECs. In the renal IRI-AKI model, the area under the curve (AUC) of TM significantly decreased both in the renal cortical and medullary after 2 h of reperfusion compared with the Sham group (p < 0.05). Normalized intensity difference (NID) of TM at different reperfusion time was all higher than that of blank microbubbles (BM) and the Sham group (p < 0.05). Ultrasound molecular imaging of TM could detect AKI early before commonly used renal function markers, histopathological biomarkers, and BM imaging. AUC of TM was negatively correlated with serum creatinine (Scr), blood urea nitrogen (BUN), and Cystatin C (Cys-C) levels, and NID of TM was linearly correlated with VCAM-1, TNF-α, and interleukin-6 (IL-6) expression (p < 0.05).
Ultrasound molecular imaging based on TM carrying VCAM-1 polypeptide can accurately evaluate the changes in renal microcirculatory perfusion and inflammatory response, which might be a promising modality for early diagnosis of AKI.